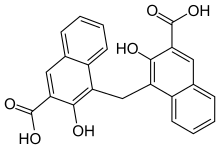
Pamoic acid, also called embonic acid, is a 2-Naphthoic acid derivative. Salts and esters of pamoic acid are known as pamoates or embonates. It can be prepared by the reaction of 3-hydroxy-2-naphthoic acid with formaldehyde.
 | |
| Names | |
|---|---|
| Preferred IUPAC name 4,4′-Methylenebis(3-hydroxynaphthalene-2-carboxylic acid) | |
| Other names 4,4′-Methylenebis(3-hydroxy-2-naphthoic acid) Embonic acid | |
| Identifiers | |
3D model (JSmol) | |
| 901319 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.545 |
| EC Number |
|
| MeSH | Pamoic+acid |
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C23H16O6 | |
| Molar mass | 388.375 g·mol−1 |
| Melting point | ≥300 °C |
| log P | 6.169 |
| Acidity (pKa) | 2.675 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | Causes skin irritation Causes serious eye irritation |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
In pharmacology, the salt form of pamoic acid (pamoate ion) can be used as a counterion of a drug compound to affect the dissolution rate of the drug.[3] The presence of multiple oxygen atoms enables significant hydrogen bonding to occur. Hydrogen bonds facilitate the dissolution of compounds in water. Pharmaceutical drugs formulated this way include cycloguanil pamoate, hydroxyzine pamoate, imipramine pamoate, olanzapine pamoate hydrate, oxantel pamoate, pyrantel pamoate, and pyrvinium pamoate.
Pamoic acid has agonist activity for the orphan G protein-coupled receptor GPR35 by which it activates ERK and beta-arrestin2, and causes antinociceptive activity.[4][5]