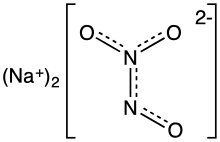
Angeli's salt, sodium trioxodinitrate, is the inorganic compound with the formula Na2[N2O3]. It contains nitrogen in an unusual reduced state. It is a colorless, water-soluble solid, hence a salt. In research, this salt is used as a source of the metastable nitroxyl (HNO), which is a signalling molecule in nature.[1] It is also known by the name sodium trioxodinitrate(II) monohydrate.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) | |
PubChem CID | |
| UNII | |
| |
| |
| Properties | |
| N2Na2O3 | |
| Molar mass | 121.991 g·mol−1 |
| Appearance | white solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Preparation and properties
As first reported by Angelo Angeli in 1896, the salt is prepared by combining hydroxylamine and an organic nitrate, as a source of nitronium (NO+
2):[2][3]
- NH2OH + RONO2 + 2 NaOR′ → ROH + 2 R′OH + Na2N2O3
The structure of the hydrate has been confirmed by X-ray crystallography. The anion is planar. Starting from the ONN end, the bond distances are 1.35 Å (N−O), 1.26 Å (N−N), 1.31 Å (N−O), and 1.32 Å (N−-O). The negative charge is on the oxygen atoms at opposite ends of the molecule. The angles are 112.9° (Osingle−N−N), 118.4° (N−N−Otrans), and 122.5° (N−N−Ocis). This means that the nitrogen–nitrogen bond is a double bond, and that the cis oxygen is slightly repelled by the single oxygen.[4]
Reaction of Angeli's salt with secondary amines in the presence of a proton source results in extrusion of N2 via isodiazenes as proposed intermediates.[5]