Complement component 3, often simply called C3, is a protein of the immune system that is found primarily in the blood. It plays a central role in the complement system of vertebrate animals and contributes to innate immunity. In humans it is encoded on chromosome 19 by a gene called C3.[5][6]
Deficiencies and defects of C3 result in the affected person being immunocompromised and particularly vulnerable to bacterial infections.
Function
C3 plays a central role in the activation of the complement system.[7] Its activation is required for both classical and alternative complement activation pathways. People with C3 deficiency are susceptible to bacterial infection.[8][9]
One form of C3-convertase, also known as C4b2a, is formed by a heterodimer of activated forms of C4 and C2. It catalyzes the proteolytic cleavage of C3 into C3a and C3b, generated during activation through the classical pathway as well as the lectin pathway. C3a is an anaphylotoxin and the precursor of some cytokines such as ASP, and C3b serves as an opsonizing agent. Factor I can cleave C3b into C3c and C3d, the latter of which plays a role in enhancing B cell responses. In the alternative complement pathway, C3 is cleaved by C3bBb, another form of C3-convertase composed of activated forms of C3 (C3b) and factor B (Bb). Once C3 is activated to C3b, it exposes a reactive thioester that allows the peptide to covalently attach to any surface that can provide a nucleophile such as a primary amine or a hydroxyl group. Activated C3 can then interact with factor B. Factor B is then activated by factor D, to form Bb. The resultant complex, C3bBb, is called the alternative pathway (AP) C3 convertase.
C3bBb is deactivated in steps. First, the proteolytic component of the convertase, Bb, is removed by complement regulatory proteins having decay-accelerating factor (DAF) activity. Next, C3b is broken down progressively to first iC3b, then C3c + C3dg, and then finally C3d. Factor I is the protease cleaves C3b but requires a cofactor (e.g Factor H, CR1, MCP or C4BP) for activity.
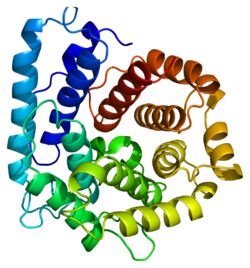
Structure
Several crystallographic structures of C3 have been determined[10] and reveal that this protein contains 13 domains.[11][12][13][14]
The C3 precursor protein is first processed by the removal of 4 Arginine residues, forming two chains, beta and alpha, linked by a disulfide bond. The C3 convertase activates C3 by cleaving the alpha chain, releasing C3a anaphylatoxin and generating C3b (beta chain + alpha' (alpha prime) chain).
Biochemistry
Biosynthesis
In humans, C3 is predominantly synthesised by liver hepatocytes[5] and to some degree by epidermis keratinocytes.[15]
Clinical use
| C4 (C) | FB (A) | C3 | CH50 | Conditions |
|---|---|---|---|---|
| · | ↓ | ↓ | ↓ | PSG, C3 NeF AA |
| ↓ | · | ↓ | · | HAE, C4D |
| · | · | · | ↓ | TCPD |
| ↓ | ·/↓ | ↓ | ↓ | SLE |
| ↑ | ↑ | ↑ | ↑ | inflammation |
Levels of C3 in the blood may be measured to support or refute a particular medical diagnosis. For example, low C3 levels are associated with Systemic Lupus Erythematosus (SLE)[16]and some types of kidney disease such as post-infectious glomerulonephritis, membranoproliferative glomerulonephritis, and shunt nephritis.
Regulation
Factor H is the primary regulator of C3. Deficiency of Factor H may lead to uncontrolled C3 activity through the alternative pathway of the complement system.[17]
Pathology
Deficiency of C3 results in the affected person being immunocompromised. Specifically, they are vulnerable to bacterial pathogens, including repeat infections by the same organism, but are not susceptible to viruses. This vulnerability also occurs in an individual deficient in C1, C2, C4, or any of their required components or associated proteins, and the clinical effects are very similar regardless of the specific deficiency. This is because all of these must work with C3 for the complement system to function.[18]
Affected people are particularly vulnerable to infections with Gram-negative organisms such as pathogenic E. coli or Salmonella enterica.[19] Additionally, C3 and other complement deficiencies are associated with frequent and severe respiratory infections, as well as other infections that invade and penetrate tissue layers.[18]
Some data shows that acquired C3 deficiency, including when this is intentionally done for medical immunosuppression purposes, may not significantly impact a person's immune function long-term.[20] However, by contrast, congenital C3 deficiency is known to cause chronic illness.[18]
Additionally, several forms of C3 deficiency contribute to the development of systemic lupus erythematosus and other autoimmune diseases.[18]
References
Further reading
- Bradley DT, Zipfel PF, Hughes AE (Jun 2011). "Complement in age-related macular degeneration: a focus on function". Eye. 25 (6): 683–93. doi:10.1038/eye.2011.37. PMC 3178140. PMID 21394116.
External links
- GeneReviews/NCBI/NIH/UW entry on Atypical Hemolytic-Uremic Syndrome
- OMIM entries on Atypical Hemolytic-Uremic Syndrome
- GeneReviews/NCBI/NIH/UW entry on Dense Deposit Disease/Membranoproliferative Glomerulonephritis Type II
- Complement+C3 at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P01024 (Complement C3) at the PDBe-KB.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.