Glycineamide ribonucleotide (or GAR) is a biochemical intermediate in the formation of purine nucleotides via inosine-5-monophosphate, and hence is a building block for DNA and RNA.[1][2][3] The vitamins thiamine[4] and cobalamin[5] also contain fragments derived from GAR.[6]
 | |
| Names | |
|---|---|
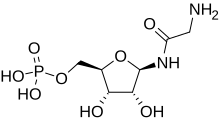
| IUPAC name (1R)-1,4-Anhydro-1-glycinamido-D-ribitol 5-(dihydrogen phosphate) | |
| Systematic IUPAC name [(2R,3S,4R,5R)-5-(2-Aminoacetamido)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate | |
| Other names Glycineamide ribotide, GAR | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C7H15N2O8P | |
| Molar mass | 286.177 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
GAR is the product of the enzyme phosphoribosylamine—glycine ligase acting on phosphoribosylamine (PRA) to combine it with glycine in a process driven by ATP. The reaction, EC 6.3.4.13 forms an amide bond:[7]
- PRA + glycine + ATP → GAR + ADP + Pi
The biosynthesis pathway next adds a formyl group from 10-formyltetrahydrofolate to GAR, catalysed by phosphoribosylglycinamide formyltransferase in reaction EC 2.1.2.2 and producing formylglycinamide ribotide (FGAR):[7]
- GAR + 10-formyltetrahydrofolate → FGAR + tetrahydrofolate
See also
References
🔥 Top keywords: Main PageSpecial:SearchWikipedia:Featured picturesYasukeHarrison ButkerRobert FicoBridgertonCleopatraDeaths in 2024Joyce VincentXXXTentacionHank AdamsIt Ends with UsYouTubeNew Caledonia2024 Indian general electionHeeramandiDarren DutchyshenSlovakiaKingdom of the Planet of the ApesAttempted assassination of Robert FicoLawrence WongBaby ReindeerXXX: Return of Xander CageThelma HoustonFuriosa: A Mad Max SagaMegalopolis (film)Richard GaddKepler's SupernovaWicked (musical)Sunil ChhetriXXX (2002 film)Ashley MadisonAnya Taylor-JoyPlanet of the ApesNava MauYoung SheldonPortal:Current eventsX-Men '97
