Osmium(IV) chloride or osmium tetrachloride is the inorganic compound composed of osmium and chlorine with the empirical formula OsCl4. It exists in two polymorphs (crystalline forms). The compound is used to prepare other osmium complexes.
 | |
| Names | |
|---|---|
| IUPAC name Osmium(IV) chloride | |
| Other names Osmium chloride, osmium tetrachloride | |
| Identifiers | |
3D model (JSmol) | |
| ECHA InfoCard | 100.151.226 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| OsCl4 | |
| Molar mass | 332.041 g/mol |
| Appearance | red-black orthorhombic crystals |
| Density | 4.38 g/cm3 |
| Melting point | decomposes at 323°C |
| reacts with water | |
| Solubility | soluble in hydrochloric acid |
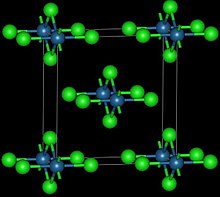
| Structure | |
| Orthorhombic, oS10 | |
| Cmmm, No. 65 | |
| Related compounds | |
Other anions | Osmium tetrabromide |
Other cations | Iron(III) chloride Ruthenium(III) chloride Osmium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Preparation, structure, reactions
It was first reported in 1909 as the product of chlorination of osmium metal.[1]This route affords the high temperature polymorph:[2]
- Os + 2 Cl2 → OsCl4
This reddish-black polymorph is orthorhombic and adopts a structure in which osmium centres are octahedrally coordinated, sharing opposite edges of the OsCl6 octahedra to form a chain.[3] A brown, apparently cubic polymorph forms upon reduction of osmium tetroxide with thionyl chloride:[4]
- OsO4 + 2 SOCl2 → OsCl4 + 2 Cl2 + 2 SO2
Osmium tetraoxide dissolves in hydrochloric acid to give the hexachloroosmate anion:
- OsO4 + 10 HCl → H2OsCl6 + 2 Cl2 + 4 H2O
References
🔥 Top keywords: Main PageSpecial:SearchWikipedia:Featured picturesYasukeHarrison ButkerRobert FicoBridgertonCleopatraDeaths in 2024Joyce VincentXXXTentacionHank AdamsIt Ends with UsYouTubeNew Caledonia2024 Indian general electionHeeramandiDarren DutchyshenSlovakiaKingdom of the Planet of the ApesAttempted assassination of Robert FicoLawrence WongBaby ReindeerXXX: Return of Xander CageThelma HoustonFuriosa: A Mad Max SagaMegalopolis (film)Richard GaddKepler's SupernovaWicked (musical)Sunil ChhetriXXX (2002 film)Ashley MadisonAnya Taylor-JoyPlanet of the ApesNava MauYoung SheldonPortal:Current eventsX-Men '97