Sulfamide (IUPAC name: sulfuric diamide) is a compound with the chemical formula SO2(NH2)2 and structure H2N−S(=O)2−NH2. Sulfamide is produced by the reaction of sulfuryl chloride with ammonia. Sulfamide was first prepared in 1838 by the French chemist Henri Victor Regnault.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name Sulfuric diamide | |
| Preferred IUPAC name Sulfamide | |
| Other names Sulphamide Sulfuryl amide | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.029.330 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| H4N2O2S | |
| Molar mass | 96.11 g/mol |
| Appearance | White orthorhombic plates |
| Melting point | 93 °C (199 °F; 366 K) |
| Boiling point | 250 °C (482 °F; 523 K) (decomposes) |
| Freely soluble | |
| -44.4×10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Sulfamide functional group
In organic chemistry, the term sulfamide may also refer to the functional group which consists of at least one organic group attached to a nitrogen atom of sulfamide.
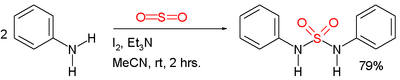
Symmetric sulfamides can be prepared directly from amines, sulfur dioxide gas and an oxidant:[3]
In this example, the reactants are aniline, triethylamine (Et3N, Et = ethyl group), and iodine. Sulfur dioxide is believed to be activated through a series of intermediates: Et3N−+−I−, Et3N−I+−I−3 and Et3N+−SO−2.
The sulfamide functional group is an increasingly common structural feature used in medicinal chemistry.[4]