Ligand Types on Electron Counting
Main article: Electron counting
Electron counting in organometallic chemistry based on ligand type involves assigning a specific number of electrons to a ligand based on its electron-donating ability. Different ligands (L, X, or Z) contribute varying numbers of electrons to the overall electron count of the organometallic complex.[1]
- Main-Group Ligands (X-Type Ligands):
- Alkyls (e.g., CH₃, C₂H₅): Each alkyl group is usually considered as one electron donor.
- Aryls (e.g., Ph, C₆H₅): Similar to alkyls, each aryl group is typically considered as one electron donor.
- Hydrides (H⁻): Each hydride ion contributes one electron.
- π-Donor Ligands (L-Type Ligands):
- Olefins (e.g., ethylene, C₂H₄): Each π-bonded pair of electrons is usually counted as one electron donor.
- Dienes (e.g., butadiene, C₄H₆): Each π-bonded pair of electrons is counted.
- π-Acceptor Ligands (Z-Type Ligands):
- Carbon Monoxide (CO): Each CO ligand is considered a two-electron donor due to the presence of a π bond.
- Isocyanides (e.g., RNC): Similar to CO, isocyanides are considered as two-electron donors.
- Neutral Ligands (e.g., H2, N2, O2):
- Neutral ligands may or may not contribute electrons to the electron count, depending on their bonding nature.
There are exceptions to these general rules.
Hapticity
Main article: Hapticity
Hapticity (represented by greek letter η) refers to the number of contiguous atoms that comprise a donor site and attach to a metal center. The η-notation applies when multiple atoms are coordinated. For example, η2 is a ligand that coordinates through two contiguous atoms. Note that Butadiene forms both η2 and η4 complexes depending on the number of carbon atoms that are bonded to the metal.[3][4][5]
Ligand exchange
A ligand exchange (also called ligand substitution) is a type of chemical reaction in which a ligand in a compound is replaced by another. One type of pathway for substitution is the ligand dependent pathway. In organometallic chemistry, this can take place via associative substitution or by dissociative substitution, which are pure pathways compared to interchange mechanisms such as cross-coupling and olefin metathesis.[6][7]
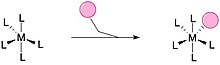
Associative substitution is a common method for ligands to saturate an undercoordinated metal complex. This pathway closely resembles the SN2 mechanism in organic chemistry. A typically smaller ligand can attach to an uncoordinated metal complex to fully saturate the complex, complete the metal's 18-electron count and lose another ligand on the metal complex. Common method for square planar complexes and when the over-coordinated intermediate is stabilized.[6][8]

Contrarily, dissociative substitution is common for ligands in a coordinately saturated metal complex. This pathway closely resembles the SN1 mechanism in organic chemistry. A typically bulkier ligand can dissociate from a coordinately saturated metal complex if the under-coordinated intermediate is stabilized. This is a common method for octahedral complexes.[6][8]
Many cross-coupling reaction exchange ligands via transmetalation, a process that typically involves the transfer of a ligand from one metal to another.[9]
General explanation of ligand exchange transmetalation:
- Start with one organometallic complex (Complex 1) containing a metal (M1) coordinated to ligands (L1). Complex 1: M1(L1)n
- There is another organometallic complex (Complex 2) with a different metal (M2) coordinated to different ligands (L2). Complex 2: M2(L2)m
- Ligand Exchange Transmetalation Reaction: In the ligand exchange transmetalation, both the metal and the ligands are exchanged between the two complexes, resulting in new organometallic complexes: M1(L1)n + M2(L2)m ⟶ M1(L2)m + M2(L1)n The metals M1 and M2 are exchanged, and the ligands L1 and L2 are also exchanged between the two complexes.
- The outcome of the ligand exchange transmetalation is two new organometallic complexes where both the metal and ligands have been switched between Complex 1 and Complex 2.

The Suzuki cross-coupling reaction facilitates the exchange of ligands between an organo-palladium complex and an organo-borano species.[11] Another example is the Stille reaction, where ligand exchange occurs between an organo-palladium complex and organo-tin species.[12]
Olefin metathesis is the redistribution of fragments of alkenes, known as olefins in organic reactions, by breaking and then regenerating the carbon-carbon double bond. Catalysts such as Grubbs catalyst, apart of the N-heterocyclic carbene family, drives this reaction in order to break the initial carbon-carbon double bond and can also drive ring openings, making olefin metathesis possible for cyclic compounds.[13][14]