The membrane (M) protein (previously called E1, sometimes also matrix protein[2]) is an integral membrane protein that is the most abundant of the four major structural proteins found in coronaviruses.[3][4][5] The M protein organizes the assembly of coronavirus virions through protein-protein interactions with other M protein molecules as well as with the other three structural proteins, the envelope (E), spike (S), and nucleocapsid (N) proteins.[4][6][7][8]
| Membrane protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
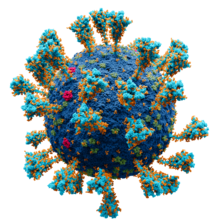 Model of the external structure of the SARS-CoV-2 virion.[1] ● Blue: envelope ● Turquoise: spike glycoprotein (S) ● Red: envelope proteins (E) ● Green: membrane proteins (M) ● Orange: glycans | |||||||||
| Identifiers | |||||||||
| Symbol | CoV_M | ||||||||
| Pfam | PF01635 | ||||||||
| InterPro | IPR002574 | ||||||||
| PROSITE | PS51927 | ||||||||
| |||||||||
Structure
The M protein is a transmembrane protein with three transmembrane domains and is around 230 amino acid residues long.[8][9] In SARS-CoV-2, the causative agent of COVID-19, the M protein is 222 residues long.[10] Its membrane topology orients the C-terminus toward the cytosolic face of the membrane and thus into the interior of the virion. It has a short N-terminal segment and a larger C-terminal domain. Although the protein sequence is not well conserved across all coronavirus groups, there is a conserved amphipathic region near the C-terminal end of the third transmembrane segment.[8][9]
M functions as a homodimer.[4][5] Studies of the M protein in multiple coronaviruses by cryo-electron microscopy have identified two distinct functional protein conformations, thought to have different roles in forming protein-protein interactions with other structural proteins.[5] M protein of SARS-CoV-2 is homologous to the prokaryotic sugar transport protein SemiSWEET.[11]
Post-translational modifications
M is a glycoprotein whose glycosylation varies according to coronavirus subgroup; N-linked glycosylation is typically found in the alpha and gamma groups while O-linked glycosylation is typically found in the beta group.[8][9] There are some exceptions; for example, in SARS-CoV, a betacoronavirus, the M protein has one N-glycosylation site.[8][6] Glycosylation state does not appear to have a measurable effect on viral growth.[6][9][12] No other post-translational modifications have been described for the M protein.[4]
Expression and localization
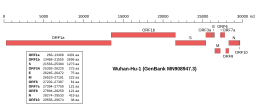 Genomic organisation of isolate Wuhan-Hu-1, the earliest sequenced sample of SARS-CoV-2, indicating the location of the M gene | |
| NCBI genome ID | 86693 |
|---|---|
| Genome size | 29,903 bases |
| Year of completion | 2020 |
| Genome browser (UCSC) | |
The gene encoding the M protein is located toward the 3' end of the virus's positive-sense RNA genome, along with the genes for the other three structural proteins and various virus-specific accessory proteins.[6][8] M is translated by membrane-bound polysomes[6] to be inserted into the endoplasmic reticulum (ER) and trafficked to the endoplasmic reticulum-Golgi intermediate compartment (ERGIC), the intracellular compartment that gives rise to the coronavirus viral envelope, or to the Golgi apparatus.[8][7][6] The exact localization is dependent on the specific virus protein.[13] Investigations of the subcellular localization of the MERS-CoV M protein found C-terminal sequence signals associated with trafficking to the Golgi.[14]
Function

The M protein is the most abundant protein in coronavirus virions.[8][5][4] It is essential for viral replication.[4]
Viral assembly
The primary function of the M protein is organizing assembly of new virions.[4] It is involved in establishing viral shape and morphology. Individual M molecules interact with each other to form the viral envelope[7][9][8] and may be able to exclude host cell proteins from the viral membrane.[5] Studies of the SARS-CoV M protein suggest that M-M interactions involve both the N- and C-termini.[6] Coronaviruses are moderately pleomorphic and conformational variations of M appear to be associated with virion size.[5]
M forms protein-protein interactions with all three other major structural proteins.[4][7] M is necessary but not sufficient for viral assembly; M and the E protein expressed together are reportedly sufficient to form virus-like particles,[7] though some reports vary depending on experimental conditions and the specific virus studied.[6][13] In some reports M appears to be capable of inducing membrane curvature,[5] though others report M alone is insufficient for this and E is required.[7] Although the E protein is not necessarily essential, it appears to be required for normal viral morphology and may be responsible for establishing curvature or initiating viral budding.[7] M also appears to have functional roles in the later stages of viral maturation, secretion, and budding.[4]
Incorporation of the spike protein (S) - which is required for assembly of infectious virions - is reported to occur though M interactions and may depend on specific conformations of M.[5][13] The conserved amphipathic region C-terminal to the third transmembrane segment is important for spike interactions.[13] Interactions with M appear to be required for correct subcellular localization of S at the viral budding site.[12] M interacts directly with the nucleocapsid (N) protein without requiring the presence of RNA.[6] This interaction appears to occur primarily through both proteins' C-termini.[4]
Interactions with the immune system

The M protein in MERS-CoV, SARS-CoV, and SARS-CoV-2 has been described as an antagonist of interferon response.[4][17]
The M protein is immunogenic and has been reported to be a determinant of humoral immunity.[4] Cytotoxic T cell responses to M have been described.[16] Antibodies to epitopes found in the M protein have been identified in patients recovered from severe acute respiratory syndrome (SARS).[18]
Other recent research has identified that SAS-COV-2 membrane protein when treated on human PBMC's causes a significant increase in pro inflammatory mediators such as TNF and IL-6.[19] The effects of exogenous SARS-COV-2 membrane protein challenge in mice was also studied. In these studies, exogenous membrane protein treated intra nasally caused a significant increase in pulmonary inflammation in mice leading to histological changes within the lungs.[20]
Host cell entry
It has been reported that human coronavirus NL63 relies on the M protein as well as the S protein to mediate host cell interactions preceding viral entry. M is thought to bind heparan sulfate proteoglycans exposed on the cell surface.[21]
Evolution and conservation
A study of SARS-CoV-2 sequences collected during the COVID-19 pandemic found that missense mutations in the M gene were relatively uncommon and suggested it was under purifying selection.[22] Similar results have been described for broader population genetics analyses over a wider range of related viruses, finding that the sequences of M and several non-structural proteins in the coronavirus genome are most subject to evolutionary constraints.[23]