Melatonin, an indoleamine, is a natural compound produced by various organisms, including bacteria and eukaryotes.[1] Its discovery in 1958 by Aaron B. Lerner and colleagues stemmed from the isolation of a substance from the pineal gland of cows that could induce skin lightening in common frogs. This compound was later identified as a hormone secreted in the brain during the night, playing a crucial role in regulating the sleep-wake cycle, also known as the circadian rhythm, in vertebrates.[2][3]
 | |
 | |
| Names | |
|---|---|
| IUPAC name N-[2-(5-methoxy-1H-indol-3-yl)ethyl]acetamide | |
| Other names 5-Methoxy-N-acetyltryptamine; N-Acetyl-5-methoxytryptamine; NSC-113928 | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.725 |
| EC Number |
|
| KEGG | |
| MeSH | Melatonin |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C13H16N2O2 | |
| Molar mass | 232.281 g/mol |
| Melting point | 117 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
In vertebrates, melatonin's functions extend to synchronizing sleep-wake cycles, encompassing sleep-wake timing and blood pressure regulation, as well as controlling seasonal rhythmicity (circannual cycle), which includes reproduction, fattening, molting, and hibernation.[4] Its effects are mediated through the activation of melatonin receptors and its role as an antioxidant.[5][6][7] In plants and bacteria, melatonin primarily serves as a defense mechanism against oxidative stress, indicating its evolutionary significance.[8] The mitochondria, key organelles within cells, are the main producers of antioxidant melatonin,[9] underscoring the molecule's "ancient origins" and its fundamental role in protecting the earliest cells from reactive oxygen species.[10][11]
In addition to its endogenous functions as a hormone and antioxidant, melatonin is also administered exogenously as a dietary supplement and medication. It is utilized in the treatment of sleep disorders, including insomnia and various circadian rhythm sleep disorders.
Biological activity
In humans, melatonin primarily acts as a potent full agonist of two types of melatonin receptors: melatonin receptor 1, with picomolar binding affinity, and melatonin receptor 2, with nanomolar binding affinity. Both receptors are part of the G-protein coupled receptors (GPCRs) family, specifically the Gi/o alpha subunit GPCRs,[12][13] although melatonin receptor 1 also exhibits coupling with Gq alpha subunit.[12]
Furthermore, melatonin functions as a high-capacity antioxidant, or free radical scavenger, within mitochondria, playing a dual role in combating cellular oxidative stress. Firstly, it directly neutralizes free radicals, and secondly it promotes the gene expression of essential antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase. This increase in antioxidant enzyme expression is mediated through signal transduction pathways activated by the binding of melatonin to its receptors. Through these mechanisms, melatonin protects the cell against oxidative stress in two ways, and plays other roles in human health than only regulating the sleep-wake cycle.[14][12][15][16][17][18]
Biological functions
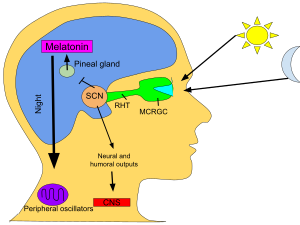
Circadian rhythm
In mammals, melatonin is critical for the regulation of sleep–wake cycles, or circadian rhythms.[19] The establishment of regular melatonin levels in human infants occurs around the third month after birth, with peak concentrations observed between midnight and 8:00 am.[20] It has been documented that melatonin production diminishes as a person ages.[21] Additionally, a shift in the timing of melatonin secretion is observed during adolescence, resulting in delayed sleep and wake times, increasing their risk for delayed sleep phase disorder during this period.[22]
The antioxidant properties of melatonin were first recognized in 1993.[23] In vitro studies reveal that melatonin directly neutralizes various reactive oxygen species, including hydroxyl (OH•), superoxide (O2−•), and reactive nitrogen species such as nitric oxide (NO•).[24][25] In plants, melatonin works synergistically with other antioxidants, enhancing the overall effectiveness of each antioxidant.[25] This compound has been found to be twice as efficacious as vitamin E, a known potent lipophilic antioxidant, in combating oxidative stress.[26] The promotion of antioxidant enzyme expression, such as superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase, is mediated through melatonin receptor-triggered signal transduction pathways.[12][14]
Melatonin's concentration in the mitochondrial matrix is significantly higher than that found in the blood plasma,[15][16][17] emphasizing its role not only in direct free radical scavenging but also in modulating the expression of antioxidant enzymes and maintaining mitochondrial integrity. This multifaceted role shows the physiological significance of melatonin as a mitochondrial antioxidant, a notion supported by numerous scholars.[14][15][16][17][18]
Furthermore, the interaction of melatonin with reactive oxygen and nitrogen species results in the formation of metabolites capable of reducing free radicals.[12][18] These metabolites, including cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), and N1-acetyl-5-methoxykynuramine (AMK), contribute to the broader antioxidative effects of melatonin through further redox reactions with free radicals.[12][18]
Immune system
Melatonin's interaction with the immune system is recognized, yet the specifics of these interactions remain inadequately defined.[27][28][needs update] An anti-inflammatory effect appears to be the most significant.[citation needed] The efficacy of melatonin in disease treatment has been the subject of limited trials, with most available data deriving from small-scale, preliminary studies. It is posited that any beneficial immunological impact is attributable to melatonin's action on high-affinity receptors (MT1 and MT2), which are present on immunocompetent cells. Preclinical investigations suggest that melatonin may augment cytokine production and promote the expansion of T cells,[29] thereby potentially mitigating acquired immunodeficiencies.[30]
Weight regulation
Melatonin's potential to regulate weight gain is posited to involve its inhibitory effect on leptin, a hormone that serves as a long-term indicator of the body's energy status.[31][32] Leptin is important for regulating energy balance and body weight by signaling satiety and reducing food intake. Melatonin, by modulating leptin's actions outside of waking hours, may contribute to the restoration of leptin sensitivity during daytime, thereby counteracting leptin resistance.
Biochemistry
Biosynthesis

The biosynthesis of melatonin in animals involves a sequence of enzymatic reactions starting with L-tryptophan, which can be synthesized through the shikimate pathway from chorismate, found in plants, or obtained from protein catabolism. The initial step in the melatonin biosynthesis pathway is the hydroxylation of L-tryptophan's indole ring by the enzyme tryptophan hydroxylase, resulting in the formation of 5-hydroxytryptophan (5-HTP). Subsequently, 5-HTP undergoes decarboxylation, facilitated by pyridoxal phosphate and the enzyme 5-hydroxytryptophan decarboxylase, yielding serotonin.[33]
Serotonin, an essential neurotransmitter, is further converted into N-acetylserotonin by the action of serotonin N-acetyltransferase, utilizing acetyl-CoA.[34] The final step in the pathway involves the methylation of N-acetylserotonin's hydroxyl group by hydroxyindole O-methyltransferase, with S-adenosyl methionine as the methyl donor, to produce melatonin.[34]
In bacteria, protists, fungi, and plants, the synthesis of melatonin also involves tryptophan as an intermediate but originates indirectly from the shikimate pathway. The pathway commences with D-erythrose 4-phosphate and phosphoenolpyruvate, and in photosynthetic cells, additionally involves carbon dioxide. While the subsequent biosynthetic reactions share similarities with those in animals, there are slight variations in the enzymes involved in the final stages.[35][36]
The hypothesis that melatonin synthesis occurs within mitochondria and chloroplasts suggests an evolutionary and functional significance of melatonin in cellular energy metabolism and defense mechanisms against oxidative stress, reflecting the molecule's ancient origins and its multifaceted roles across different domains of life.[37]
Mechanism

The mechanism of melatonin biosynthesis initiates with the hydroxylation of L-tryptophan, a process that requires the cofactor tetrahydrobiopterin (THB) to react with oxygen and the active site iron of tryptophan hydroxylase. Although the complete mechanism is not entirely understood, two main mechanisms have been proposed:
The first mechanism involves a slow transfer of one electron from THB to molecular oxygen (O2), potentially producing a superoxide (O−2). This superoxide could then recombine with the THB radical to form 4a-peroxypterin. 4a-peroxypterin may either react with the active site iron (II) to create an iron-peroxypterin intermediate or directly transfer an oxygen atom to the iron, facilitating the hydroxylation of L-tryptophan.
Alternatively, the second mechanism proposes that oxygen interacts with the active site iron (II) first, forming iron (III) superoxide. This molecule could then react with THB to form an iron-peroxypterin intermediate.
Following the formation of iron (IV) oxide from the iron-peroxypterin intermediate, this oxide selectively attacks a double bond to yield a carbocation at the C5 position of the indole ring. A subsequent 1,2-shift of the hydrogen and the loss of one of the two hydrogen atoms on C5 would restore aromaticity, producing 5-hydroxy-L-tryptophan.[38]
The decarboxylation of 5-hydroxy-L-tryptophan to produce 5-hydroxytryptamine is then facilitated by a decarboxylase enzyme with pyridoxal phosphate (PLP) as a cofactor.[39] PLP forms an imine with the amino acid derivative, facilitating the breaking of the carbon–carbon bond and release of carbon dioxide. The protonation of the amine derived from tryptophan restores the aromaticity of the pyridine ring, leading to the production of 5-hydroxytryptamine and PLP.[40]
Serotonin N-acetyltransferase, with histidine residue His122, is hypothesized to deprotonate the primary amine of 5-hydroxytryptamine. This deprotonation allows the lone pair on the amine to attack acetyl-CoA, forming a tetrahedral intermediate. The thiol from coenzyme A then acts as a leaving group when attacked by a general base, producing N-acetylserotonin.[41]
The final step in the biosynthesis of melatonin involves the methylation of N-acetylserotonin at the hydroxyl position by SAM, resulting in the production of S-adenosyl homocysteine (SAH) and melatonin.[40][42]
Regulation
In vertebrates, the secretion of melatonin is regulated through the activation of the beta-1 adrenergic receptor by the hormone norepinephrine.[43] Norepinephrine increases the concentration of intracellular cAMP via beta-adrenergic receptors, which in turn activates the cAMP-dependent protein kinase A (PKA). PKA then phosphorylates arylalkylamine N-acetyltransferase (AANAT), the penultimate enzyme in the melatonin synthesis pathway. When exposed to daylight, noradrenergic stimulation ceases, leading to the immediate degradation of the protein by proteasomal proteolysis.[44] The production of melatonin recommences in the evening, a phase known as the dim-light melatonin onset.
Blue light, especially within the 460–480 nm range, inhibits the biosynthesis of melatonin,[45] with the degree of suppression being directly proportional to the intensity and duration of light exposure. Historically, humans in temperate climates experienced limited exposure to blue daylight during winter months, primarily receiving light from sources that emitted predominantly yellow light, such as fires.[46] The incandescent light bulbs used extensively throughout the 20th century emitted relatively low levels of blue light.[47] It has been found that light containing only wavelengths greater than 530 nm does not suppress melatonin under bright-light conditions.[48] The use of glasses that block blue light in the hours preceding bedtime can mitigate melatonin suppression.[49] Additionally, wearing blue-blocking goggles during the last hours before bedtime is recommended for individuals needing to adjust to an earlier bedtime since melatonin facilitates the onset of sleep.[50]
Metabolism
Melatonin is metabolized with an elimination half-life ranging from 20 to 50 minutes.[51][2][52] The primary metabolic pathway transforms melatonin into 6-hydroxymelatonin, which is then conjugated with sulfate and excreted in urine as a waste product.[53]
Measurement
For both research and clinical purposes, melatonin levels in humans can be determined through saliva or blood plasma analysis.[54]
Use as a medication and supplement
Melatonin is used both as a prescription medication and an over-the-counter dietary supplement for the management of sleep disorders, including insomnia and various circadian rhythm sleep disorders such as delayed sleep phase disorder, jet lag disorder, and shift work sleep disorder.[55] In addition to melatonin, a range of synthetic melatonin receptor agonists, namely ramelteon, tasimelteon, and agomelatine, are used in medicine.[56][57]
A study published by the Journal of the American Medical Association (JAMA) in April 2023 found that a 12% of the 30 melatonin product preparations analyzed had melatonin quantities within ±10% of the amounts specified on their labels. Some supplements were found to contain up to 347% of the declared melatonin content. In Europe, melatonin is classified as an active pharmaceutical ingredient, highlighting the regulatory oversight of its use and distribution. Conversely, as of 2022[update], the United States was considering the inclusion of melatonin in pharmacy compounding practices. A preceding study from 2022 concluded that consuming unregulated melatonin products can expose individuals, including children, to melatonin quantities ranging from 40 to 130 times higher than the recommended levels when products are used 'as directed'.[58]
History
Discovery
Melatonin's discovery is linked to the study of skin color changes in some amphibians and reptiles, a phenomenon initially observed through the administration of pineal gland extracts.[59][60] In 1917, Carey Pratt McCord and Floyd P. Allen found that feeding extracts from the pineal glands of cows caused the skin of tadpoles to lighten by contracting the dark epidermal melanophores.[61][62]
The hormone melatonin was isolated in 1958 by Aaron B. Lerner, a dermatology professor, and his team at Yale University. Motivated by the possibility that a substance from the pineal gland could be beneficial in treating skin diseases, they extracted and identified melatonin from bovine pineal gland extracts.[63] Subsequent research in the mid-1970s by Lynch and others demonstrated that melatonin production follows a circadian rhythm in human pineal glands.[64]
The first patent for the therapeutic use of melatonin as a low-dose sleep aid was awarded to Richard Wurtman at the Massachusetts Institute of Technology in 1995.[65]
Etymology
The etymology of melatonin stems from its skin-lightening properties. As detailed in their publication in the Journal of the American Chemical Society,[66] Lerner and his colleagues proposed the name melatonin, derived from the Greek words melas, meaning 'black' or 'dark', and tonos, meaning 'labour',[67] 'colour'[68] or 'suppress'.[69] This naming convention follows that of serotonin, another agent affecting skin color, discovered in 1948 as a modulator of vascular tone, which influenced its name based on its serum vasoconstrictor effect.[70] Melatonin was thus aptly named to reflect its role in preventing the darkening of the skin, highlighting the intersection of biochemistry and linguistics in scientific discovery.[66]
Occurrence
Animals
In vertebrates, melatonin is produced in darkness, thus usually at night, by the pineal gland, a small endocrine gland[71]located in the center of the brain but outside the blood–brain barrier. Light/dark information reaches the suprachiasmatic nuclei from retinal photosensitive ganglion cells of the eyes[72][73] rather than the melatonin signal (as was once postulated). Known as "the hormone of darkness", the onset of melatonin at dusk promotes activity in nocturnal (night-active) animals and sleep in diurnal ones including humans.[74]
Many animals use the variation in duration of melatonin production each day as a seasonal clock.[75] In animals including humans,[76] the profile of melatonin synthesis and secretion is affected by the variable duration of night in summer as compared to winter. The change in duration of secretion thus serves as a biological signal for the organization of daylength-dependent (photoperiodic) seasonal functions such as reproduction, behavior, coat growth, and camouflage coloring in seasonal animals.[76] In seasonal breeders that do not have long gestation periods and that mate during longer daylight hours, the melatonin signal controls the seasonal variation in their sexual physiology, and similar physiological effects can be induced by exogenous melatonin in animals including mynah birds[77] and hamsters.[78] Melatonin can suppress libido by inhibiting secretion of luteinizing hormone and follicle-stimulating hormone from the anterior pituitary gland, especially in mammals that have a breeding season when daylight hours are long. The reproduction of long-day breeders is repressed by melatonin and the reproduction of short-day breeders is stimulated by melatonin.
During the night, melatonin regulates leptin, lowering its levels.
Cetaceans have lost all the genes for melatonin synthesis as well as those for melatonin receptors.[79] This is thought to be related to their unihemispheric sleep pattern (one brain hemisphere at a time). Similar trends have been found in sirenians.[79]
Plants
Until its identification in plants in 1987, melatonin was for decades thought to be primarily an animal neurohormone. When melatonin was identified in coffee extracts in the 1970s, it was believed to be a byproduct of the extraction process. Subsequently, however, melatonin has been found in all plants that have been investigated. It is present in all the different parts of plants, including leaves, stems, roots, fruits, and seeds, in varying proportions.[8][80] Melatonin concentrations differ not only among plant species, but also between varieties of the same species depending on the agronomic growing conditions, varying from picograms to several micrograms per gram.[36][81] Notably high melatonin concentrations have been measured in popular beverages such as coffee, tea, wine, and beer, and crops including corn, rice, wheat, barley, and oats.[8] In some common foods and beverages, including coffee[8] and walnuts,[82] the concentration of melatonin has been estimated or measured to be sufficiently high to raise the blood level of melatonin above daytime baseline values.
Although a role for melatonin as a plant hormone has not been clearly established, its involvement in processes such as growth and photosynthesis is well established. Only limited evidence of endogenous circadian rhythms in melatonin levels has been demonstrated in some plant species and no membrane-bound receptors analogous to those known in animals have been described. Rather, melatonin performs important roles in plants as a growth regulator, as well as environmental stress protector. It is synthesized in plants when they are exposed to both biological stresses, for example, fungal infection, and nonbiological stresses such as extremes of temperature, toxins, increased soil salinity, drought, etc.[36][83][84]
Herbicide-induced oxidative stress has been experimentally mitigated in vivo in a high-melatonin transgenic rice.[85][86]
Fungal disease resistance is another role. Added melatonin increases resistance in Malus prunifolia against Diplocarpon mali.[86][87] Also acts as a growth inhibitor on fungal pathogens including Alternaria, Botrytis, and Fusarium spp. Decreases the speed of infection. As a seed treatment, protects Lupinus albus from fungi. Dramatically slows Pseudomonas syringae tomato DC3000 infecting Arabidopsis thaliana and infecting Nicotiana benthamiana.[87]
Fungi
Melatonin has been observed to reduce stress tolerance in Phytophthora infestans in plant-pathogen systems.[88] Danish pharmaceutical company Novo Nordisk have used genetically modified yeast (Saccharomyces cerevisiae) to produce melatonin.[89]
Bacteria
Melatonin is produced by α-proteobacteria and photosynthetic cyanobacteria. There is no report of its occurrence in archaea which indicates that melatonin originated in bacteria[11] most likely to prevent the first cells from the damaging effects of oxygen in the primitive Earth's atmosphere.[10]
Novo Nordisk have used genetically modified Escherichia coli to produce melatonin.[90][91]
Food products
Naturally-occurring melatonin has been reported in foods including tart cherries to about 0.17–13.46 ng/g,[92] bananas, plums, grapes, rice, cereals, herbs,[93] olive oil, wine,[94] and beer.[95] The consumption of milk and sour cherries may improve sleep quality.[96] When birds ingest melatonin-rich plant feed, such as rice, the melatonin binds to melatonin receptors in their brains.[97] When humans consume foods rich in melatonin, such as banana, pineapple, and orange, the blood levels of melatonin increase significantly.[98]
References
External links

- "Melatonin". Chemwatch.
