| Nonmetals in their periodic table context |
 |
| usually/always counted as a nonmetal[1][2][3] |
| sometimes counted as a nonmetal[4][a] |
| status as nonmetal or metal unconfirmed[5] |
Nonmetals are chemical elements that mostly lack distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter (less dense) than metals; brittle or crumbly if solid; and often poor conductors of heat and electricity. Chemically, nonmetals have high electronegativity (meaning they usually attract electrons in a chemical bond); and their oxides tend to be acidic.
Seventeen elements are widely recognized as nonmetals. Additionally, some or all of six borderline elements (metalloids) are sometimes counted as nonmetals.
The two lightest nonmetals, hydrogen and helium, together make up about 98% of the mass of the observable universe. Five nonmetallic elements—hydrogen, carbon, nitrogen, oxygen, and silicon—make up the bulk of Earth's oceans, atmosphere, biosphere, and crust.
The diverse properties of nonmetals enable a range of natural and technological uses. Hydrogen, oxygen, carbon, and nitrogen are essential building blocks for life. Industrial uses of nonmetals include electronics, energy storage, agriculture, and chemical production.
Most nonmetallic elements were not identified until the 18th and 19th centuries. While a distinction between metals and other minerals had existed since antiquity, a basic classification of chemical elements as metallic or nonmetallic emerged only in the late 18th century. Since then over two dozen properties have been suggested as criteria for distinguishing nonmetals from metals.
Definition and applicable elements
- Unless otherwise noted, this article describes the most stable form of an element in ambient conditions.[b]

Nonmetallic chemical elements are generally described as lacking properties common to metals, namely shininess, pliability, good thermal and electrical conductivity, and a general capacity to form basic oxides.[7][8] There is no widely-accepted precise definition;[9] any list of nonmetals is open to debate and revision.[1] The elements included depend on the properties regarded as most representative of nonmetallic or metallic character.
Fourteen elements are almost always recognized as nonmetals:[1][2]
Three more are commonly classed as nonmetals, but some sources list them as "metalloids",[3] a term which refers to elements regarded as intermediate between metals and nonmetals:[10]
One or more of the six elements most commonly recognized as metalloids are sometimes instead counted as nonmetals:
About 15–20% of the 118 known elements[11] are thus classified as nonmetals.[c]
General properties
Physical
of some nonmetallic elements
Nonmetals vary greatly in appearance, being colorless, colored or shiny.For the colorless nonmetals (hydrogen, nitrogen, oxygen, and the noble gases), their electrons are held sufficiently strongly so that no absorption of light happens in the visible part of the spectrum, and all visible light is transmitted.[14]The colored nonmetals (sulfur, fluorine, chlorine, bromine) absorb some colors (wavelengths) and transmit the complementary or opposite colors. For example, chlorine's "familiar yellow-green colour ... is due to a broad region of absorption in the violet and blue regions of the spectrum".[15][d] The shininess of boron, graphitic carbon, silicon, black phosphorus, germanium, arsenic, selenium, antimony, tellurium, and iodine[e] is a result of their structures featuring varying degrees of delocalized (free-moving) electrons that scatter incoming visible light.[18]
About half of nonmetallic elements are gases; most of the rest are solids. Bromine, the only liquid, is so volatile that it is usually topped by a layer of its fumes. The gaseous and liquid nonmetals have very low densities, melting and boiling points, and are poor conductors of heat and electricity.[19] The solid nonmetals have low densities and low mechanical and structural strength (being brittle or crumbly),[20] and a wide range of electrical conductivity.[f]
This diversity in form stems from variability in internal structures and bonding arrangements. Nonmetals existing as discrete atoms like xenon, or as small molecules, such as oxygen, sulfur, and bromine, have low melting and boiling points; many are gases at room temperature, as they are held together by weak London dispersion forces acting between their atoms or molecules.[24] In contrast, nonmetals that form giant structures, such as chains of up to 1,000 selenium atoms,[25] sheets of carbon atoms in graphite,[26] or three-dimensional lattices of silicon atoms[27] have higher melting and boiling points, and are all solids, as it takes more energy to overcome their stronger covalent bonds.[28] Nonmetals closer to the left or bottom of the periodic table (and so closer to the metals) often have some weak metallic interactions between their molecules, chains, or layers; this occurs in boron,[29] carbon,[30] phosphorus,[31] arsenic,[32] selenium,[33] antimony,[34] tellurium[35] and iodine.[36]
| Aspect | Metals | Nonmetals |
|---|---|---|
| Appearance and form | Shiny if freshly prepared or fractured; few colored;[37] all but one solid[38] | Shiny, colored or transparent;[39] all but one solid or gaseous[38] |
| Density | Often higher | Often lower |
| Elasticity | Mostly malleable and ductile | Brittle if solid |
| Electrical conductivity[40] | Good | Poor to good |
| Electronic structure[41] | Metallic or semimetalic | Semimetallic, semiconductor, or insulator |
The structures of nonmetallic elements differ from those of metals primarily due to variations in valence electron numbers and atomic size. Metals typically have fewer valence electrons than available orbitals, leading them to share electrons with many nearby atoms, resulting in centrosymmetrical crystalline structures.[42] In contrast, nonmetals share only the electrons required to achieve a noble gas electron configuration.[43] For example, nitrogen forms diatomic molecules featuring a triple bonds between each atom, both of which thereby attain the configuration of the noble gas neon. Antimony's larger atomic size prevents triple bonding, resulting in buckled layers in which each antimony atom is singly bonded with three other nearby atoms.[44]
The electrical and thermal conductivities of nonmetals, along with the brittle nature of solid nonmetals, are likewise related to their internal arrangements. Whereas good conductivity and plasticity (malleability, ductility) are ordinarily associated with the presence of free-moving and evenly distributed electrons in metals,[45] the electrons in nonmetals typically lack such mobility.[46] Among nonmetallic elements, good electrical and thermal conductivity is seen only in carbon (as graphite, along its planes), arsenic, and antimony.[g] Good thermal conductivity otherwise occurs only in boron, silicon, phosphorus, and germanium;[21] such conductivity is transmitted though vibrations of the crystalline lattices of these elements.[47] Moderate electrical conductivity is observed in the semiconductors[48] boron, silicon, phosphorus, germanium, selenium, tellurium, and iodine. Plasticity occurs under limited circumstances in carbon, as seen in exfoliated (expanded) graphite[49][50] and carbon nanotube wire,[51] in white phosphorus (soft as wax, pliable and can be cut with a knife, at room temperature),[52] in plastic sulfur,[53] and in selenium which can be drawn into wires from its molten state.[54]
The physical differences between metals and nonmetals arise from internal and external atomic forces. Internally, the positive charge stemming from the protons in an atom's nucleus acts to hold the atom's outer electrons in place. Externally, the same electrons are subject to attractive forces from protons in neighboring atoms. When the external forces are greater than, or equal to, the internal force, the outer electrons are expected to become relatively free to move between atoms, and metallic properties are predicted. Otherwise nonmetallic properties are expected.[55]
Allotropes
Over half of the nonmetallic elements exhibit a range of less stable allotropic forms, each with distinct physical properties.[56] For example, carbon, the most stable form of which is graphite, can manifest as diamond, buckminsterfullerene,[57] and amorphous[58] and paracrystalline (mixed amorphous and crystalline)[59] variations. Allotropes also occur for nitrogen, oxygen, phosphorus, sulfur, selenium, the six metalloids, and iodine.[60]
Chemical

| Aspect | Metals | Nonmetals | |
|---|---|---|---|
| Reactivity[61] | Wide range: very reactive to noble | ||
| Oxides | lower | Basic | Acidic; never basic[62] |
| higher | Increasingly acidic | ||
| Compounds with metals[63] | Alloys | Ionic compounds | |
| Ionization energy[64] | Low to high | Moderate to very high | |
| Electronegativity[65] | Low to high | Moderate to very high | |
Nonmetals have relatively high values of electronegativity, and their oxides are therefore usually acidic. Exceptions may occur if a nonmetal is not very electronegative, or if its oxidation state is low, or both. These non-acidic oxides of nonmetals may be amphoteric (like water, H2O[66]) or neutral (like nitrous oxide, N2O[67][h]), but never basic (as is common with metals).
Nonmetals tend to gain or share electrons during chemical reactions, in contrast to metals which tend to donate electrons. This behavior is closely related to the stability of electron configurations in the noble gases, which have complete outer shells. Nonmetals generally gain enough electrons to attain the electron configuration of the following noble gas, while metals tend to lose electrons, in some cases achieving the electron configuration of the preceding noble gas. These tendencies in nonmetallic elements are succinctly summarized by the duet and octet rules of thumb.[70]
They typically exhibit higher ionization energies, electron affinities, and standard electrode potentials than metals. Generally, the higher these values are (including electronegativity) the more nonmetallic the element tends to be.[71] For example, the chemically very active nonmetals fluorine, chlorine, bromine, and iodine have an average electronegativity of 3.19—a figure[i] higher than that of any individual metal. On the other hand, the 2.05 average of the chemically weak metalloid nonmetals[j] falls within the 0.70 to 2.54 range of metals.[65]
The chemical distinctions between metals and nonmetals primarily stem from the attractive force between the positive nuclear charge of an individual atom and its negatively charged outer electrons. From left to right across each period of the periodic table, the nuclear charge increases in tandem with the number of protons in the atomic nucleus.[72] Consequently, there is a corresponding reduction in atomic radius[73] as the heightened nuclear charge draws the outer electrons closer to the nucleus core.[74] In metals, the impact of the nuclear charge is generally weaker compared to nonmetallic elements. As a result, in chemical bonding, metals tend to lose electrons, leading to the formation of positively charged ions or polarized atoms, while nonmetals tend to gain these electrons due to their stronger nuclear charge, resulting in negatively charged ions or polarized atoms.[75]
The number of compounds formed by nonmetals is vast.[76] The first 10 places in a "top 20" table of elements most frequently encountered in 895,501,834 compounds, as listed in the Chemical Abstracts Service register for November 2, 2021, were occupied by nonmetals. Hydrogen, carbon, oxygen, and nitrogen collectively appeared in most (80%) of compounds. Silicon, a metalloid, ranked 11th. The highest-rated metal, with an occurrence frequency of 0.14%, was iron, in 12th place.[77] A few examples of nonmetal compounds are: boric acid (H
3BO
3), used in ceramic glazes;[78] selenocysteine (C
3H
7NO
2Se), the 21st amino acid of life;[79] phosphorus sesquisulfide (P4S3), found in strike anywhere matches;[80] and teflon ((C
2F
4)n), used to create non-stick coatings for pans and other cookware.[81]
Complications
Adding complexity to the chemistry of the nonmetals are anomalies occurring in the first row of each periodic table block; non-uniform periodic trends; higher oxidation states; multiple bond formation; and property overlaps with metals.
First row anomaly

| Condensed periodic table highlighting the first row of each block: s p d and f | |||||||||||||
| Period | s-block | ||||||||||||
| 1 | H 1 | He 2 | p-block | ||||||||||
| 2 | Li 3 | Be 4 | B 5 | C 6 | N 7 | O 8 | F 9 | Ne 10 | |||||
| 3 | Na 11 | Mg 12 | d-block | Al 13 | Si 14 | P 15 | S 16 | Cl 17 | Ar 18 | ||||
| 4 | K 19 | Ca 20 | Sc-Zn 21-30 | Ga 31 | Ge 32 | As 33 | Se 34 | Br 35 | Kr 36 | ||||
| 5 | Rb 37 | Sr 38 | f-block | Y-Cd 39-48 | In 49 | Sn 50 | Sb 51 | Te 52 | I 53 | Xe 54 | |||
| 6 | Cs 55 | Ba 56 | La-Yb 57-70 | Lu-Hg 71-80 | Tl 81 | Pb 82 | Bi 83 | Po 84 | At 85 | Rn 86 | |||
| 7 | Fr 87 | Ra 88 | Ac-No 89-102 | Lr-Cn 103-112 | Nh 113 | Fl 114 | Mc 115 | Lv 116 | Ts 117 | Og 118 | |||
| Group | (1) | (2) | (3-12) | (13) | (14) | (15) | (16) | (17) | (18) | ||||
| The first-row anomaly strength by block is s >> p > d > f.[82][k] | |||||||||||||
Starting with hydrogen, the first row anomaly primarily arises from the electron configurations of the elements concerned. Hydrogen is particularly notable for its diverse bonding behaviors. It most commonly forms covalent bonds, but it can also lose its single electron in an aqueous solution, leaving behind a bare proton with tremendous polarizing power.[83] Consequently, this proton can attach itself to the lone electron pair of an oxygen atom in a water molecule, laying the foundation for acid-base chemistry.[84] Moreover, a hydrogen atom in a molecule can form a second, albeit weaker, bond with an atom or group of atoms in another molecule. Such bonding, "helps give snowflakes their hexagonal symmetry, binds DNA into a double helix; shapes the three-dimensional forms of proteins; and even raises water's boiling point high enough to make a decent cup of tea."[85]
Hydrogen and helium, as well as boron through neon, have unusually small atomic radii. This phenomenon arises because the 1s and 2p subshells lack inner analogues (meaning there is no zero shell and no 1p subshell), and they therefore experience no electron repulsion effects, unlike the 3p, 4p, and 5p subshells of heavier elements.[86] As a result, ionization energies and electronegativities among these elements are higher than what periodic trends would otherwise suggest. The compact atomic radii of carbon, nitrogen, and oxygen facilitate the formation of double or triple bonds.[87]
While it would normally be expected, on electron configuration consistency grounds, that hydrogen and helium would be placed atop the s-block elements, the significant first row anomaly shown by these two elements justifies alternative placements. Hydrogen is occasionally positioned above fluorine, in group 17, rather than above lithium in group 1. Helium is commonly placed above neon, in group 18, rather than above beryllium in group 2.[88]
Secondary periodicity
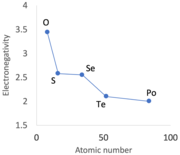
An alternation in certain periodic trends, sometimes referred to as secondary periodicity, becomes evident when descending groups 13 to 15, and to a lesser extent, groups 16 and 17.[89][l] Immediately after the first row of d-block metals, from scandium to zinc, the 3d electrons in the p-block elements—specifically, gallium (a metal), germanium, arsenic, selenium, and bromine—prove less effective at shielding the increasing positive nuclear charge. This same effect is observed with the emergence of fourteen f-block metals located between barium and lutetium, ultimately leading to atomic radii that are smaller than expected for elements from hafnium (Hf) onward.[91]
The Soviet chemist Shchukarev gives two more tangible examples:[92]
- "The toxicity of some arsenic compounds, and the absence of this property in analogous compounds of phosphorus [P] and antimony [Sb]; and the ability of selenic acid [H2SeO4] to bring metallic gold [Au] into solution, and the absence of this property in sulfuric [H2SO4] and [H2TeO4] acids."
Higher oxidation states
Some nonmetallic elements exhibit oxidation states that deviate from those predicted by the octet rule, which typically results in a valency of –3 in group 15, –2 in group 16, –1 in group 17, and 0 in group 18. Examples of such states can include compounds like ammonia (NH3), hydrogen sulfide (H2S), hydrogen fluoride (HF), and elemental xenon (Xe). Meanwhile, the maximum possible oxidation state increases from +5 in group 15, to +8 in group 18. The +5 oxidation state is observable from period 2 onward, in compounds such as nitric acid (HNO3) and phosphorus pentafluoride (PCl5).[m] Higher oxidation states in later groups emerge from period 3 onwards, as seen in sulfur hexafluoride (SF6), iodine heptafluoride (IF7), and xenon tetroxide (XeO4). For heavier nonmetals, their larger atomic radii and lower electronegativity values enable the formation of compounds with higher oxidation numbers, supporting higher bulk coordination numbers.[93]
Multiple bond formation
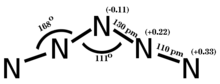
Period 2 nonmetals, particularly carbon, nitrogen, and oxygen, show a propensity to form multiple bonds. The compounds formed by these elements often exhibit unique stoichiometries and structures, as seen in the various nitrogen oxides,[93] which are not commonly found in elements from later periods.
Property overlaps
While certain elements have traditionally been classified as nonmetals and others as metals, some overlapping of properties occurs. Writing early in the twentieth century, by which time the era of modern chemistry had been well-established,[95] Humphrey[96] observed that:
- ... these two groups, however, are not marked off perfectly sharply from each other; some nonmetals resemble metals in certain of their properties, and some metals approximate in some ways to the non-metals.

Examples of metal-like properties occurring in nonmetallic elements include:
- silicon has an electronegativity (1.9) comparable with metals such as cobalt (1.88), copper (1.9), nickel (1.91) and silver (1.93);[65]
- the electrical conductivity of graphite exceeds that of some metals;[o]
- selenium can be drawn into a wire;[54]
- radon is the most metallic of the noble gases and begins to show some cationic behavior, which is unusual for a nonmetal;[100] and
- just over half of nonmetallic elements can form homopolyatomic cations.[p]
Examples of nonmetal-like properties occurring in metals are:
- Tungsten displays some nonmetallic properties, being brittle, having a high electronegativity, and forming only anions in aqueous solution,[102] and predominately acidic oxides.[8][103] These are characteristics more aligned with nonmetals. Even so, tungsten is classified as a metal, illustrating the spectrum of behaviors elements can exhibit within their classifications.
- Gold, the "king of metals" demonstrates several nonmetallic behaviors. It has the highest electrode potential among metals, suggesting a preference for gaining rather than losing electrons. Gold's ionization energy is one of the highest among metals, and its electron affinity and electronegativity are high, with the latter exceeding that of some nonmetals. It forms the Au– auride anion and exhibits a tendency to bond to itself, behaviors which are unexpected for metals. In aurides (MAu, where M = Li–Cs), gold's behavior is similar to that of a halogen, thereby bridging the traditional metal-nonmetal divide.[104]
A relatively recent development involves certain compounds of heavier p-block elements, such as silicon, phosphorus, germanium, arsenic and antimony, exhibiting behaviors typically associated with transition metal complexes. This phenomenon is linked to a small energy gap between their filled and empty molecular orbitals, which are the regions in a molecule where electrons reside and where they can be available for chemical reactions. In such compounds, this closer energy alignment allows for unusual reactivity with small molecules like hydrogen (H2), ammonia (NH3), and ethylene (C2H4), a characteristic previously observed primarily in transition metal compounds. These reactions may open new avenues in catalytic applications.[105]
Types
Nonmetal classification schemes vary widely, with some accommodating as few as two subtypes and others identifying up to seven. For example, the periodic table in the Encyclopaedia Britannica recognizes noble gases, halogens, and other nonmetals, and splits the elements commonly recognized as metalloids between "other metals" and "other nonmetals".[106] On the other hand, seven of twelve color categories on the Royal Society of Chemistry periodic table include nonmetals.[107][q]
| Group (1, 13−18) | Period | ||||||
| 13 | 14 | 15 | 16 | 1/17 | 18 | (1−6) | |
| H | He | 1 | |||||
| B | C | N | O | F | Ne | 2 | |
| Si | P | S | Cl | Ar | 3 | ||
| Ge | As | Se | Br | Kr | 4 | ||
| Sb | Te | I | Xe | 5 | |||
| Rn | 6 | ||||||
Starting on the right side of the periodic table, three types of nonmetals can be recognized:
The elements in a fourth set are sometimes recognized as nonmetals:
While many of the early workers attempted to classify elements none of their classifications were satisfactory. They were divided into metals and nonmetals, but some were soon found to have properties of both. These were called metalloids. This only added to the confusion by making two indistinct divisions where one existed before.[128]
Whiteford & Coffin 1939, Essentials of College Chemistry
The boundaries between these types are not sharp.[v] Carbon, phosphorus, selenium, and iodine border the metalloids and show some metallic character, as does hydrogen.
The greatest discrepancy between authors occurs in metalloid "frontier territory".[130] Some consider metalloids distinct from both metals and nonmetals, while others classify them as nonmetals.[4] Some categorize certain metalloids as metals (e.g., arsenic and antimony due to their similarities to heavy metals).[131][w] Metalloids resemble the elements universally considered "nonmetals" in having relatively low densities, high electronegativity, and similar chemical behavior.[127][x]
For context, the metallic side of the periodic table also ranges widely in reactivity.[y] Highly reactive metals fill most of the s- and f-blocks on the left,[z] bleeding into the early part of the d-block. Thereafter, reactivity generally decreases closer to the p-block, whose metals are not particularly reactive.[aa] The very unreactive noble metals, such as platinum and gold, are clustered in an island within the d-block.[137]
Noble gases

Six nonmetals are classified as noble gases: helium, neon, argon, krypton, xenon, and the radioactive radon. In conventional periodic tables they occupy the rightmost column. They are called noble gases due to their exceptionally low chemical reactivity.[108]
These elements exhibit remarkably similar properties, characterized by their colorlessness, odorlessness, and nonflammability. Due to their closed outer electron shells, noble gases possess feeble interatomic forces of attraction, leading to exceptionally low melting and boiling points.[138] As a consequence, they all exist as gases under standard conditions, even those with atomic masses surpassing many typically solid elements.[139]
Chemically, the noble gases exhibit relatively high ionization energies, negligible or negative electron affinities, and high to very high electronegativities. The number of compounds formed by noble gases is in the hundreds and continues to expand,[140] with most of these compounds involving the combination of oxygen or fluorine with either krypton, xenon, or radon.[141]
Halogen nonmetals
While the halogen nonmetals are notably reactive and corrosive elements, they can also be found in everyday compounds like toothpaste (NaF); common table salt (NaCl); swimming pool disinfectant (NaBr); and food supplements (KI). The term "halogen" itself means "salt former".[142]
Physically, fluorine and chlorine exist as pale yellow and yellowish-green gases, respectively, while bromine is a reddish-brown liquid, typically covered by a layer of its fumes; iodine is a solid and under white light is metallic-looking.[143] Electrically, the first three elements function as insulators while iodine behaves as a semiconductor (along its planes).[144]
Chemically, the halogen nonmetals exhibit high ionization energies, electron affinities, and electronegativity values, and are mostly relatively strong oxidizing agents.[145] These characteristics contribute to their corrosive nature.[146] All four elements tend to form primarily ionic compounds with metals,[147] in contrast to the remaining nonmetals (except for oxygen) which tend to form primarily covalent compounds with metals.[ab] The highly reactive and strongly electronegative nature of the halogen nonmetals epitomizes nonmetallic character.[151]
Unclassified nonmetals

After classifying the nonmetallic elements into noble gases and halogens, but before encountering the metalloids, there are seven nonmetals: hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur, and selenium.
In their most stable forms, three of these are colorless gases (hydrogen, nitrogen, oxygen); three are metallic looking solids (carbon, phosphorus, selenium); and one is a yellow solid (sulfur). Electrically, graphitic carbon behaves as a semimetal along its planes[153] and a semiconductor perpendicular to its planes;[154] phosphorus and selenium are semiconductors;[155] while hydrogen, nitrogen, oxygen, and sulfur are insulators.[ac]
These elements are often considered too diverse to merit a collective name,[157] and have been referred to as other nonmetals,[158] or simply as nonmetals.[159] As a result, their chemistry is typically taught disparately, according to their respective periodic table groups:[160] hydrogen in group 1; the group 14 nonmetals (including carbon, and possibly silicon and germanium); the group 15 nonmetals (including nitrogen, phosphorus, and possibly arsenic and antimony); and the group 16 nonmetals (including oxygen, sulfur, selenium, and possibly tellurium). Authors may choose other subdivisions based on their preferences.[ad]
Hydrogen, in particular, behaves in some respects like a metal and in others like a nonmetal.[162] Like a metal it can, for example, form a solvated cation in aqueous solution;[163] it can substitute for alkali metals in compounds such as the chlorides (NaCl cf. HCl) and nitrates (KNO3 cf. HNO3), and in certain alkali metal organometallic structures;[164] and it can form alloy-like hydrides with some transition metals.[165] Conversely, it is an insulating diatomic gas, akin to the nonmetals nitrogen, oxygen, fluorine and chlorine. In chemical reactions, it tends to ultimately attain the electron configuration of helium (the following noble gas) behaving in this way as a nonmetal.[166] It attains this configuration by forming a covalent or ionic bond[167] or, if it has initially given up its electron, by attaching itself to a lone pair of electrons.[168]
Some or all of these nonmetals share several properties. Being generally less reactive than the halogens,[169] most of them can occur naturally in the environment.[170] They have significant roles in biology[171] and geochemistry.[157] Collectively, their physical and chemical characteristics can be described as "moderately non-metallic".[157] However, they all have corrosive aspects. Hydrogen can corrode metals. Carbon corrosion can occur in fuel cells.[172] Acid rain is caused by dissolved nitrogen or sulfur. Oxygen causes iron to corrode via rust. White phosphorus, the most unstable form, ignites in air and leaves behind phosphoric acid residue.[173] Untreated selenium in soils can lead to the formation of corrosive hydrogen selenide gas.[174] When combined with metals, the unclassified nonmetals can form high-hardness (interstitial or refractory) compounds[175] due to their relatively small atomic radii and sufficiently low ionization energies.[157] They also exhibit a tendency to bond to themselves, particularly in solid compounds.[176] Additionally, diagonal periodic table relationships among these nonmetals mirror similar relationships among the metalloids.[177]
Metalloids
The six elements more commonly recognized as metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium, all of which have a metallic appearance. (Other elements appearing less commonly on lists of metalloids include carbon, aluminium, selenium and polonium; these have both metallic and nonmetallic properties, but one or the other predominates.) In the periodic table, metalloids occupy a diagonal region within the p-block extending from boron at the upper left to tellurium at the lower right, along the dividing line between metals and nonmetals shown on some tables.[3]
Metalloids are brittle and poor-to-good conductors of heat and electricity. Specifically, boron, silicon, germanium, and tellurium are semiconductors. Arsenic and antimony have the electronic band structures of semimetals, although both have less stable semiconducting allotropes: arsenic as arsenolamprite, an extremely rare naturally occurring form;[178] and antimony in its synthetic thin-film amorphous form.[3][179]
Chemically, metalloids generally behave like weak nonmetals. Among the nonmetallic elements they tend to have the lowest ionization energies, electron affinities, and electronegativity values, and are relatively weak oxidizing agents. Additionally, they tend to form alloys when combined with metals.[3]
Abundance, sources, and uses
Abundance
| Universe[180] | hydrogen 70.5%, helium 27.5% | oxygen 1% |
| Atmosphere[181] | nitrogen 78%, oxygen 21% | argon 0.5% |
| Hydrosphere[181] | oxygen 66.2%, hydrogen 33.2% | chlorine 0.3% |
| Biomass[182] | oxygen 63%, carbon 20%, hydrogen 10% | nitrogen 3.0% |
| Crust[181] | oxygen 61%, silicon 20% | hydrogen 2.9% |
Hydrogen and helium dominate the observable universe, making up an estimated 98% of all ordinary matter by mass.[ae] Oxygen, the next most abundant element, accounts for about 1%.[184]
Five nonmetals—hydrogen, carbon, nitrogen, oxygen, and silicon—form the bulk of the directly observable structure of the earth: about 84% of the crust, 96% of the biomass, and over 99% of the atmosphere and hydrosphere, as shown in the accompanying table.[181][182]
The Earth's mantle and core, making up about 99% of the Earth's volume,[185] are estimated to be made up of oxygen (31% by weight) and silicon (16%), with the remainder largely composed of the metals iron (31%), magnesium (15%) and nickel (2%).[186][af]
Sources
| Group (1, 13−18) | Period | ||||||
| 13 | 14 | 15 | 16 | 1/17 | 18 | (1−6) | |
| H | He | 1 | |||||
| B | C | N | O | F | Ne | 2 | |
| Si | P | S | Cl | Ar | 3 | ||
| Ge | As | Se | Br | Kr | 4 | ||
| Sb | Te | I | Xe | 5 | |||
| Rn | 6 | ||||||
Nonmetals and metalloids are extracted from a variety of raw materials:[170]
Uses
| Nearly all nonmetals have uses in:[189][190] Household goods, lighting and lasers, and medicine and pharmaceuticals |
| Most nonmetals have uses in:[189][191] Agrochemicals, dyestuffs and smart phones |
| Some nonmetals have uses in or as:[189][192] Alloys, cryogenics and refrigerants, explosives, fire retardants, fuel cells, inert air replacements, insulation (thermal & electric), mineral acids, nuclear control rods, photography, plastics, plug-in hybrid vehicles, solar cells, water treatment, welding gases, and vulcanization |
| Metalloids have uses in:[193] Alloys, ceramics, oxide glasses, solar cells, and semiconductors |
The great variety of physical and chemical properties of nonmetals[194] enable a wide range of natural and technological uses, as shown in the accompanying table. In living organisms, hydrogen, oxygen, carbon, and nitrogen serve as the foundational building blocks of life.[195] Some key technological uses of nonmetallic elements are in lighting and lasers, medicine and pharmaceuticals, and ceramics and plastics.
Some specific uses of later-discovered or rarer nonmetallic elements include:
- Boron, first produced in a pure form in 1909,[196] is used in the form of high-strength fibers for aerospace components and certain sporting goods.[197] It is also added to steel alloys to improve hardenability.[198]
- Black phosphorus, first reported in 1916,[199] is employed mainly in high-performance electronic devices, including field-effect transistors (FETs), owing to its exceptional charge carrier mobility. It has potential applications in photodetectors, optoelectronic devices, advanced solar cells and thermoelectric materials.[200]
- Germanium, thought to be a metal up until the 1930s,[201] was historically used in electronics, particularly early transistors and diodes, and still has roles in specialized high-frequency electronics. It is used in the production of infrared optical components for thermal imaging and spectroscopy.[202]
- Xenon, one of the rarest elements on Earth,[203] finds use in high-intensity discharge lamps for bright white light in automotive headlights and marine lighting. Additionally, it serves as a contrast agent in medical imaging techniques like xenon computed tomography and xenon-enhanced magnetic resonance imaging. In space exploration, xenon is a propellant for ion thrusters, known for their efficiency.[204]
- Radon, the rarest noble gas,[205] was formerly used in radiography and radiation therapy. Usually, radium in either an aqueous solution or as a porous solid was stored in a glass vessel. The radium decayed to produce radon, which was pumped off, filtered, and compressed into a small tube every few days. The tube was then sealed and removed. It was a source of gamma rays, which came from bismuth-214, one of radon's decay products.[206] In radiotherapy, radon has now been replaced by 137cesium, 192iridium, and 103palladium.[207]
History, background, and taxonomy
Discovery

While most nonmetallic elements were identified during the 18th and 19th centuries, a few were recognized much earlier. Carbon, sulfur, and antimony were known in antiquity. Arsenic was discovered in the Middle Ages (credited to Albertus Magnus) and phosphorus in 1669 (isolated from urine by Hennig Brand). Helium, identified in 1868, is the only element not initially discovered on Earth itself.[ah] The most recently identified nonmetal is radon, detected at the end of the 19th century.[170]
Some nonmetals occur naturally as free elements, others required intricate extraction or isolation procedures. Such procedures included spectroscopy, fractional distillation, radiation detection, electrolysis, ore acidification, displacement reactions, combustion, and controlled heating processes.
The noble gases, renowned for their low reactivity, were first identified via spectroscopy, air fractionation, and radioactive decay studies. Helium was initially detected by its distinctive yellow line in the solar corona spectrum. Subsequently, it was observed escaping as bubbles when uranite UO2 was dissolved in acid. Neon, argon, krypton, and xenon were obtained through the fractional distillation of air. The discovery of radon occurred three years after Henri Becquerel's pioneering research on radiation in 1896.[209]
The isolation of the halogen nonmetals from their halides involved techniques including electrolysis, acid addition, or displacement. These efforts were not without peril, as some chemists tragically[210] lost their lives in their pursuit of isolating fluorine.[211]
The unclassified nonmetals have a diverse history. Hydrogen was discovered and first described in 1671 as the product of the reaction between iron filings and dilute acids. Carbon was found naturally in forms like charcoal, soot, graphite, and diamond. Nitrogen was discovered by examining air after carefully removing oxygen. Oxygen itself was obtained by heating mercurous oxide. Phosphorus was derived from the heating of ammonium sodium hydrogen phosphate (Na(NH4)HPO4), a compound found in urine.[212] Sulfur occurred naturally as a free element, simplifying its isolation. Selenium,[ai] was first identified as a residue in sulfuric acid.[214]
Most metalloids were first isolated by heating their oxides (boron, silicon, arsenic, tellurium) or a sulfide (germanium).[170] Antimony, first obtained by heating its sulfide, stibnite, was later discovered in native form.[215]
Origin and use of the term
Although a distinction had existed between metals and other mineral substances since ancient times, it was only towards the end of the 18th century that a basic classification of chemical elements as either metallic or nonmetallic substances began to emerge. It would take another nine decades before the term "nonmetal" was widely adopted.

Around 340 BCE, in Book III of his treatise Meteorology, the ancient Greek philosopher Aristotle categorized substances found within the Earth into metals and "fossiles".[aj] The latter category included various minerals such as realgar, ochre, ruddle, sulfur, cinnabar, and other substances that he referred to as "stones which cannot be melted".[216]
Until the Middle Ages the classification of minerals remained largely unchanged, albeit with varying terminology. In the fourteenth century, the English alchemist Richardus Anglicus expanded upon the classification of minerals in his work Correctorium Alchemiae. In this text, he proposed the existence of two primary types of minerals. The first category, which he referred to as "major minerals", included well-known metals such as gold, silver, copper, tin, lead, and iron. The second category, labeled "minor minerals", encompassed substances like salts, atramenta (iron sulfate), alums, vitriol, arsenic, orpiment, sulfur, and similar substances that were not metallic bodies.[217]
The term "nonmetallic" dates back to at least the 16th century. In his 1566 medical treatise, French physician Loys de L'Aunay distinguished substances from plant sources based on whether they originated from metallic or non-metallic soils.[218]
Later, the French chemist Nicolas Lémery discussed metallic and nonmetallic minerals in his work Universal Treatise on Simple Drugs, Arranged Alphabetically published in 1699. In his writings, he contemplated whether the substance "cadmia" belonged to either the first category, akin to cobaltum (cobaltite), or the second category, exemplified by what was then known as calamine—a mixed ore containing zinc carbonate and silicate.[219]
The pivotal moment in the systematic classification of chemical elements into metallic and nonmetallic substances came in 1789 with the work of Antoine Lavoisier, a French chemist. He published the first modern list of chemical elements in his revolutionary[221] Traité élémentaire de chimie. The elements were categorized into distinct groups, including gases, metallic substances, nonmetallic substances, and earths (heat-resistant oxides).[222] Lavoisier's work gained widespread recognition and was republished in twenty-three editions across six languages within its first seventeen years, significantly advancing the understanding of chemistry in Europe and America.[223]
The widespread adoption of the term "nonmetal" followed a complex process spanning nearly nine decades. In 1811, the Swedish chemist Berzelius introduced the term "metalloids"[224] to describe nonmetallic elements, noting their ability to form negatively charged ions with oxygen in aqueous solutions.[225][226] While Berzelius' terminology gained significant acceptance,[227] it later faced criticism from some who found it counterintuitive,[226] misapplied,[228] or even invalid.[229][230] In 1864, reports indicated that the term "metalloids" was still endorsed by leading authorities,[231] but there were reservations about its appropriateness. The idea of designating elements like arsenic as metalloids had been considered.[231] By as early as 1866, some authors began preferring the term "nonmetal" over "metalloid" to describe nonmetallic elements.[232] In 1875, Kemshead[233] observed that elements were categorized into two groups: non-metals (or metalloids) and metals. He noted that the term "non-metal", despite its compound nature, was more precise and had become universally accepted as the nomenclature of choice.
Suggested distinguishing criteria
| Year | Property, type, and citation | |
|---|---|---|
| 1803 | Density and electrical conductivity[ak] | P[234] |
| 1821 | Opacity | P[235] |
| 1906 | Hydrolysis of halides | C[236] |
| 1911 | Cation formation | C[237] |
| 1927 | Goldhammer-Herzfeld metallization criterion[al] | P[239] |
| 1949 | Bulk coordination number | P[240] |
| 1956 | Minimum excitation potential | C[241] |
| 1956 | Acid-base nature of oxides | C[242] |
| 1957 | Electron configuration | A[243] |
| 1962 | Sonorousness[am] | P[244] |
| 1966 | Physical state | P[245] |
| 1969 | Melting and boiling points, electrical conductivity | P[246] |
| 1973 | Critical temperature | P[247] |
| 1977 | Sulfate formation | C[62] |
| 1977 | Oxide solubility in acids | C[248] |
| 1979 | 3D electrical conductivity | P[249] |
| 1986 | Enthalpy of vaporization | P[250] |
| 1991 | Liquid range[an] | P[251] |
| 1999 | Temperature coefficient of resistivity | P[252] |
| 1999 | Element structure (in bulk) | P[253] |
| 2000 | Configuration energy[ao] | C[254] |
| 2001 | Packing efficiency | P[255] |
| 2010 | Electrical conductivity at absolute zero | P[256] |
| 2010 | Electron band structure | A[256] |
| 2017 | Thermal conductivity | P[257] |
| 2017 | Atomic conductance[ap] | A[258] |
| Physical/Chemical/Atomic properties: | P/C/A | |
From the early 1800s, a variety of physical, chemical, and atomic properties have been suggested for distinguishing metals from nonmetals, as listed in the accompanying table. Some of the earliest recorded properties from 1803 are the (high) density and (good) electrical conductivity of metals.
In 1809, the British chemist and inventor Humphry Davy made a groundbreaking discovery that reshaped the understanding of metals and nonmetals.[259] When he isolated sodium and potassium, their low densities (floating on water!) contrasted with their metallic appearance, challenging the stereotype of metals as dense substances.[260][aq] Nevertheless, their classification as metals was firmly established by their distinct chemical properties.[262]
One of the most commonly recognized properties used in this context is the temperature coefficient of resistivity, the effect of heating on electrical resistance and conductivity. As temperature rises, the conductivity of metals decreases while that of nonmetals increases.[252] However, plutonium, carbon, arsenic, and antimony defy the norm. When plutonium (a metal) is heated within a temperature range of −175 to +125 °C its conductivity increases.[263] Similarly, despite its common classification as a nonmetal, when carbon (as graphite) is heated it experiences a decrease in electrical conductivity.[264] Arsenic and antimony, which are occasionally classified as nonmetals, show behavior similar to carbon, highlighting the complexity of the distinction between metals and nonmetals.[265]
Kneen and colleagues[266] proposed that the classification of nonmetals can be achieved by establishing a single criterion for metallicity. They acknowledged that various plausible classifications exist and emphasized that while these classifications may differ to some extent, they would generally agree on the categorization of nonmetals.
Emsley[267] pointed out the complexity of this task, asserting that no single property alone can unequivocally assign elements to either the metal or nonmetal category. Furthermore, Jones[268] emphasized that classification systems typically rely on more than two attributes to define distinct types.
Johnson[269] distinguished between metals and nonmetals on the basis of their physical states, electrical conductivity, mechanical properties, and the acid-base nature of their oxides:
- gaseous elements are nonmetals (hydrogen, nitrogen, oxygen, fluorine, chlorine and the noble gases);
- liquids (mercury, bromine) are either metallic or nonmetallic: mercury, as a good conductor, is a metal; bromine, with its poor conductivity, is a nonmetal;
- solids are either ductile and malleable, hard and brittle, or soft and crumbly:
- a. ductile and malleable elements are metals;
- b. hard and brittle elements include boron, silicon and germanium, which are semiconductors and therefore not metals; and
- c. soft and crumbly elements include carbon, phosphorus, sulfur, arsenic, antimony,[ar] tellurium and iodine, which have acidic oxides indicative of nonmetallic character.[as]
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Several authors[274] have noted that nonmetals generally have low densities and high electronegativity. The accompanying table, using a threshold of 7 g/cm3 for density and 1.9 for electronegativity (revised Pauling), shows that all nonmetals have low density and high electronegativity. In contrast, all metals have either high density or low electronegativity (or both). Goldwhite and Spielman[275] added that, "... lighter elements tend to be more electronegative than heavier ones." The average electronegativity for the elements in the table with densities less than 7 gm/cm3 (metals and nonmetals) is 1.97 compared to 1.66 for the metals having densities of more than 7 gm/cm3.
Some authors divide elements into metals, metalloids, and nonmetals, but Oderberg[276] disagrees, arguing that by the principles of categorization, anything not classified as a metal should be considered a nonmetal.
Development of types

In 1844, Alphonse Dupasquier, a French doctor, pharmacist, and chemist,[277] established a basic taxonomy of nonmetals to aid in their study. He wrote:[278]
- They will be divided into four groups or sections, as in the following:
- Organogens—oxygen, nitrogen, hydrogen, carbon
- Sulphuroids—sulfur, selenium, phosphorus
- Chloroides—fluorine, chlorine, bromine, iodine
- Boroids—boron, silicon.
Dupasquier's quartet parallels the modern nonmetal types. The organogens and sulphuroids are akin to the unclassified nonmetals. The chloroides were later called halogens.[279] The boroids eventually evolved into the metalloids, with this classification beginning from as early as 1864.[231] The then unknown noble gases were recognized as a distinct nonmetal group after being discovered in the late 1800s.[280]
His taxonomy was noted for its natural basis.[281][au] That said, it was a significant departure from other contemporary classifications, since it grouped together oxygen, nitrogen, hydrogen, and carbon.[283]
In 1828 and 1859, the French chemist Dumas classified nonmetals as (1) hydrogen; (2) fluorine to iodine; (3) oxygen to sulfur; (4) nitrogen to arsenic; and (5) carbon, boron and silicon,[284] thereby anticipating the vertical groupings of Mendeleev's 1871 periodic table. Dumas' five classes fall into modern groups 1, 17, 16, 15, and 14 to13 respectively.
Classification of metalloids

Boron and silicon were recognized early on as nonmetals[av] but arsenic, antimony, tellurium, and germanium have a more complicated history. While the suitability of arsenic being counted as a metalloid had been considered in 1864,[231] Mendeleev, in 1897, counted it and antimony as metals.[286] Although tellurium likely acquired an "ium" suffix due to its metallic appearance,[287] Mendeleev said it represented a transition between metals and nonmetals.[288] The semiconductor germanium was first regarded as a poorly conducting metal due to the presence of impurities. The understanding of it as a semiconductor, and subsequently as a metalloid, emerged in the 1930s with the development of semiconductor physics.[201]
Since the 1940s, these six elements have been increasingly, but not universally, recognized as metalloids.[289] In 1947, Linus Pauling included a reference to them in his classic[290] and influential[291] textbook General chemistry: An introduction to descriptive chemistry and modern chemical theory. He described boron, silicon, germanium, arsenic, antimony (and polonium) as "elements with intermediate properties."[292] He said they were in the center of his electronegativity scale, with values close to 2.[aw] The emergence of the semiconductor industry and solid-state electronics in the 1950s and 1960s highlighted the semiconducting properties of germanium and silicon (and boron and tellurium), reinforcing the idea that metalloids were "in-between" or "half-way" elements.[294] Writing in 1982, Goldsmith[289] observed that, "The newest approach is to emphasize aspects of their physical and/or chemical nature such as electronegativity, crystallinity, overall electronic nature and the role of certain metalloids as semiconductors."
Comparison of selected properties
The two tables in this section list some of the properties of five types of elements (noble gases, halogen nonmetals, unclassified nonmetals, metalloids and, for comparison, metals) based on their most stable forms in ambient conditions.
The aim is to show that most properties display a left-to-right progression in metallic-to-nonmetallic character or average values.[295][296] Some overlap occurs as outlier elements of each type exhibit less-distinct, hybrid-like, or atypical properties.[297][ax] These overlaps or transitional points, along with horizontal, diagonal, and vertical relationships between the elements, form part of the "great deal of information" summarized by the periodic table.[299]
The dashed lines around the columns for metalloids signify that the treatment of these elements as a distinct type can vary depending on the author, or classification scheme in use.
Physical properties by element type
Physical properties are listed in loose order of ease of their determination.
| Property | Element type | ||||
|---|---|---|---|---|---|
| Metals | Metalloids | Unc. nonmetals | Halogen nonmetals | Noble gases | |
| General physical appearance | lustrous[19] | lustrous[300] | colorless[305] | ||
| Form and density[306] | solid (Hg liquid) | solid | solid or gas | solid or gas (bromine liquid) | gas |
| often high density such as iron, lead, tungsten | low to moderately high density | low density | low density | low density | |
| some light metals including beryllium, magnesium, aluminium | all lighter than iron | hydrogen, nitrogen lighter than air[307] | helium, neon lighter than air[308] | ||
| Elasticity | mostly malleable and ductile[19] | brittle[300] | carbon, phosphorus, sulfur, selenium, brittle[ay] | iodine brittle[310] | not applicable |
| Electrical conductivity | good[az] |
|
|
| poor[bd] |
| Electronic structure[41] | metallic (beryllium, strontium, α-tin, ytterbium, bismuth are semimetals) | semimetal (arsenic, antimony) or semiconductor |
| semiconductor (I) or insulator | insulator |
Chemical properties by element type
Chemical properties are listed from general characteristics to more specific details.
| Property | Element type | ||||
|---|---|---|---|---|---|
| Metals | Metalloids | Unc. nonmetals | Halogen nonmetals | Noble gases | |
| General chemical behavior |
| weakly nonmetallic[be] | moderately nonmetallic[296] | strongly nonmetallic[315] | |
| Oxides | basic; some amphoteric or acidic[8] | amphoteric or weakly acidic[318][bf] | acidic[bg] or neutral[bh] | acidic[bi] | metastable XeO3 is acidic;[325] stable XeO4 strongly so[326] |
| few glass formers[bj] | all glass formers[328] | some glass formers[bk] | no glass formers reported | no glass formers reported | |
| ionic, polymeric, layer, chain, and molecular structures[330] | polymeric in structure[331] |
| |||
| Compounds with metals | alloys[19] or intermetallic compounds[334] | tend to form alloys or intermetallic compounds[335] | mainly ionic[147] | simple compounds in ambient conditions not known[bl] | |
| Ionization energy (kJ mol−1)[64] ‡ | low to high | moderate | moderate to high | high | high to very high |
| 376 to 1,007 | 762 to 947 | 941 to 1,402 | 1,008 to 1,681 | 1,037 to 2,372 | |
| average 643 | average 833 | average 1,152 | average 1,270 | average 1,589 | |
| Electronegativity (Pauling)[bm][65] ‡ | low to high | moderate | moderate to high | high | high (radon) to very high |
| 0.7 to 2.54 | 1.9 to 2.18 | 2.19 to 3.44 | 2.66 to 3.98 | ca. 2.43 to 4.7 | |
| average 1.5 | average 2.05 | average 2.65 | average 3.19 | average 3.3 | |
† Hydrogen can also form alloy-like hydrides[165]
‡ The labels low, moderate, high, and very high are arbitrarily based on the value spans listed in the table
See also
- CHON (carbon, hydrogen, oxygen, nitrogen)
- List of nonmetal monographs
- Metallization pressure
- Nonmetal (astrophysics)
- Period 1 elements (hydrogen, helium)
- Properties of nonmetals (and metalloids) by group
Notes
References
Citations
Bibliography
External links
 Media related to Nonmetals at Wikimedia Commons
Media related to Nonmetals at Wikimedia Commons


















