Rhodium hexafluoride, also rhodium(VI) fluoride, (RhF6) is the inorganic compound of rhodium and fluorine. A black volatile solid,[1] it is a highly reactive material, and a rare example of a rhodium(VI) compound. It is one of seventeen known binary hexafluorides.
 | |
| Names | |
|---|---|
| IUPAC name rhodium(VI) fluoride | |
| Other names rhodium hexafluoride | |
| Identifiers | |
3D model (JSmol) | |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| F6Rh | |
| Molar mass | 216.91 g/mol |
| Appearance | black crystalline solid[1] |
| Density | 3.71g/mL[2] |
| Melting point | ≈ 70 °C (158 °F; 343 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Synthesis, structure, properties
Rhodium hexafluoride is prepared by reaction of rhodium metal with an excess of elemental fluorine:[3]
- Rh + 3 F2 → RhF6
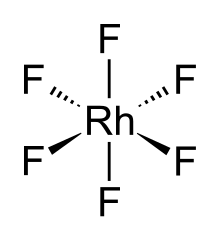
The RhF6 molecule has octahedral molecular geometry. Consistent with its d3 configuration, the six Rh–F bond lengths are equivalent, being 1.824 Å.[2] It crystallises in an orthorhombic space group Pnma with lattice parameters of a = 9.323 Å, b = 8.474 Å, and c = 4.910 Å.
Like some other metal fluorides, RhF6 is highly oxidizing. It attacks glass,[3] and can even react with elemental oxygen.[4]
References
Further reading
- Gmelins Handbuch der anorganischen Chemie, System Nr. 63, Rhodium, Part B1, pp. 266–268.
External links
 Media related to Rhodium hexafluoride at Wikimedia Commons
Media related to Rhodium hexafluoride at Wikimedia Commons- Rhodium hexafluoride at webelements.com.
