Estradiol dienanthate (EDE), sold under the brand names Climacteron among others, is a long-acting estrogen medication which was previously used in menopausal hormone therapy for women and to suppress lactation in women.[1][2][3][4] It was formulated in combination with estradiol benzoate (EB), a short-acting estrogen, and testosterone enanthate benzilic acid hydrazone (TEBH), a long-acting androgen/anabolic steroid.[2][3][4] EDE has not been made available for medical use alone.[5] The medication, in combination with EB and TEBH, was given by injection into muscle once or at regular intervals, for instance once every 6 weeks.[6][7][8]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Climacteron, Amenose, Lactimex, Lactostat (all combinations) |
| Other names | Estradiol dienantate; EDE; EDEn; E2-EDN; Estradiol diheptanoate; Estra-1,3,5(10)-triene-3,17β-diol 3,17β-diheptanoate |
| Routes of administration | Intramuscular injection |
| Drug class | Estrogen; Estrogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.028.903 |
| Chemical and physical data | |
| Formula | C32H48O4 |
| Molar mass | 496.732 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Side effects of EDE include breast tenderness, breast enlargement, nausea, headache, and fluid retention.[9] EDE is an estrogen and hence is an agonist of the estrogen receptor, the biological target of estrogens like estradiol.[10][11] It is an estrogen ester and a prodrug of estradiol in the body.[11][10] Because of this, it is considered to be a natural and bioidentical form of estrogen.[11]
EDE was first described by 1959.[12][13] It was previously available in Canada and Germany but was discontinued by 2005.[14][15][16] The medication is no longer available in any form.[5]
Medical uses
EDE, a long-acting estrogen, was used in combination with EB, a short-acting estrogen, and TEBH, a long-acting androgen/anabolic steroid, in menopausal hormone therapy in perimenopausal, postmenopausal, hypogonadal, and oophorectomized women, as well as for suppression of lactation in postpartum women.[6][8][7]
Available forms
EDE was available only in combination EB and TEBH.[17] The combination was available in two different dose forms, one for menopausal hormone therapy (brand names Climacteron, Amenose) and the other for lactation suppression (brand names Lactimex, Lactostat). Climacteron and Amenose contained 1.0 mg EB, 7.5 mg EDE, and 150 mg TEBH (69 mg free testosterone) and was given by repeated intramuscular injection at regular intervals.[6][18][8] Lactimex and Lactostat contained 6 mg EB, 15 mg EDE, and 300 mg TEBH in 2 mL of corn oil and was administered as a single intramuscular injection after childbirth or during breastfeeding.[7][19][20][21]
Pharmacology

Pharmacodynamics
EDE is an estradiol ester, or a prodrug of estradiol.[11][10] As such, it is an estrogen, or an agonist of the estrogen receptors.[11][10] EDE is of about 82% higher molecular weight than estradiol due to the presence of its C3 and C17β heptanoate (enanthate) esters. Because EDE is a prodrug of estradiol, it is considered to be a natural and bioidentical form of estrogen.[11]
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
| Estradiol enanthate | Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/d (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | |||||
- Estradiol and testosterone levels after an intramuscular injection of 1 mg estradiol benzoate, 7.5 mg estradiol dienanthate, and 150 mg testosterone enanthate benzilic acid hydrazone in oil (brand name Climacteron) in ovariectomized women.[22] Assays were performed using immunoassays.[22] Source was Sherwin (1987).[22]
- Estradiol levels after an intramuscular injection of 10 mg estradiol valerate in oil or Climacteron (1 mg estradiol benzoate, 7.5 mg estradiol dienanthate in oil) in ovariectomized women.[23][24] Assays were performed using RIA.[23][24] Source was Sherwin et al. (1987).[23][24]
Pharmacokinetics
Estradiol and testosterone levels following a single intramuscular injection of Climacteron (including 1 mg EB, 7.5 mg EDE, and 150 mg TEBH equivalent to 69 mg free testosterone) versus 10 mg estradiol valerate have been studied over 28 days.[23][24]
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
| Estradiol enanthate | Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/d (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | |||||
Chemistry
EDE is a synthetic estrane steroid and the C3 and C17β heptanoate (enanthate) diester of estradiol.[1] It is also known as estradiol 3,17β-heptanoate or as estra-1,3,5(10)-triene-3,17β-diol 3,17β-diheptanoate.[1] EDE is structurally related to estradiol enanthate (estradiol 17β-heptanoate), which has a single heptanoate ester rather than two.[1]
| Estrogen | Structure | Ester(s) | Relative mol. weight | Relative E2 contentb | log Pc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | 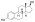 | – | – | – | – | 1.00 | 1.00 | 4.0 | |
| Estradiol acetate | 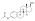 | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | |
| Estradiol benzoate |  | C3 | Benzoic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | |
| Estradiol dipropionate | 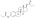 | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | |
| Estradiol valerate |  | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | |
| Estradiol benzoate butyrate | 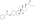 | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | |
| Estradiol cypionate | 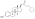 | C17β | Cyclopentylpropanoic acid | Cyclic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | |
| Estradiol enanthate | 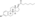 | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | |
| Estradiol dienanthate | 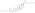 | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | |
| Estradiol undecylate |  | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | |
| Estradiol stearate |  | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | |
| Estradiol distearate |  | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | |
| Estradiol sulfate |  | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | |
| Estradiol glucuronide |  | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | |
| Estramustine phosphated |  | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | |
| Polyestradiol phosphatee |  | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | |
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic or cyclic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymer of estradiol phosphate (~13 repeat units). f = Relative molecular weight or estradiol content per repeat unit. g = log P of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
History
EDE was first described and introduced for medical use by 1959.[12][13]
Society and culture
Brand names
EDE was marketed in combination with EB and TEBH under the brand names Climacteron, Amenose, Lactimex, and Lactostat.[17][8]
Availability
EDE is no longer available but was previously used in Canada, Germany and other countries.[5][17][7][21]

