Mercury(II) sulfate, commonly called mercuric sulfate, is the chemical compound HgSO4. It is an odorless salt that forms white granules or crystalline powder. In water, it separates into an insoluble basic sulfate with a yellow color and sulfuric acid.[3]
 | |
| Names | |
|---|---|
| Other names Mercuric sulfate, Mercurypersulfate, Mercury Bisulfate[1] | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.083 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1645 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| HgSO4 | |
| Molar mass | 296.653 g/mol |
| Appearance | white monoclinic crystals |
| Odor | odorless |
| Density | 6.47 g/cm3, solid |
| 450 °C (dec.)[2] | |
| Decomposes in water to yellow mercuric subsulfate and sulfuric acid | |
| Solubility | soluble in hot H2SO4, NaCl solution insoluble in alcohol, acetone, ammonia |
| −78.1·10−6 cm3/mol | |
| Structure | |
| rhombic | |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) | −707.5 kJ mol−1[3] |
| Hazards | |
| GHS labelling:[4] | |
   | |
| Danger | |
| H300, H310, H330, H373, H410 | |
| P260, P262, P264, P270, P271, P273, P280, P284, P301+P316, P302+P352, P304+P340, P316, P319, P320, P321, P330, P361+P364, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Structure
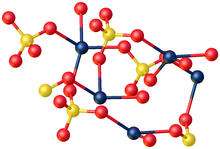
The anhydrous compound features Hg2+ in a highly distorted tetrahedral HgO4 environment. Two Hg-O distances are 2.22 Å and the others are 2.28 and 2.42 Å.[5] In the monohydrate, Hg2+ adopts a linear coordination geometry with Hg-O (sulfate) and Hg-O (water) bond lengths of 2.179 and 2.228 Å, respectively. Four weaker bonds are also observed with Hg---O distances >2.5 Å.[6]
History
In 1932, the Japanese chemical company Chisso Corporation began using mercury sulfate as the catalyst for the production of acetaldehyde from acetylene and water. Though it was unknown at the time, methylmercury is formed as side product of this reaction. Exposure and consumption of the mercury waste products, including methylmercury, that were dumped into Minamata Bay by Chisso are believed to be the cause of Minamata disease in Minamata, Japan.[7]
Production
Mercury sulfate can be producedby treating mercury with hot concentrated sulfuric acid:[8]
- Hg + 2 H2SO4 → HgSO4 + SO2 + 2 H2O
Alternatively yellow mercuric oxide reacts also with concentrated sulfuric acid.[9]
Uses
Denigés' reagent
An acidic solution of mercury sulfate is known as Denigés' reagent. It was commonly used throughout the 20th century as a qualitative analysis reagent. If Denigés' reagent is added to a solution containing compounds that have tertiary alcohols, a yellow or red precipitate will form.[10]
Hydration reactions
Mercury compounds such as mercury sulfate and mercury(II) acetate are commonly used as catalysts in oxymercuration-demercuration, a type of electrophilic addition reaction which results in hydration of an unsaturated compound. The hydration of an alkene results in an alcohol that follows the regioselectivity predicted by Markovnikov's rule. For an alkyne, the result is an enol, which tautomerizes to give a ketone.[11] An example is the conversion of 2,5-dimethylhexyne-2,5-diol to 2,2,5,5-tetramethyltetrahydrofuran using aqueous mercury sulfate without the addition of acid.[12]

As previously mentioned, HgSO4 was used as the catalyst for the production of acetaldehyde from acetylene and water.[13]
Health issues
Inhalation of HgSO4 can result in acute poisoning: causing tightness in the chest, difficulties breathing, coughing and pain. Exposure of HgSO4 to the eyes can cause ulceration of conjunctiva and cornea. If mercury sulfate is exposed to the skin it may cause sensitization dermatitis. Lastly, ingestion of mercury sulfate will cause necrosis, pain, vomiting, and severe purging. Ingestion can result in death within a few hours due to peripheral vascular collapse.[1]
It was used in the late 19th century to induce vomiting for medical reasons.[14]
References
External links

