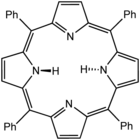
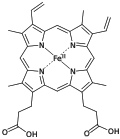
Porphyrins (/ˈpɔːrfərɪn/ POR-fər-in) are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). In vertebrates, an essential member of the porphyrin group is heme, which is a component of hemoproteins, whose functions include carrying oxygen in the bloodstream. In plants, an essential porphyrin derivative is chlorophyll, which is involved in light harvesting and electron transfer in photosynthesis.
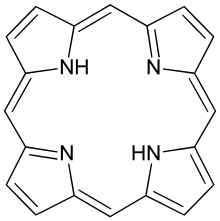
The parent of porphyrins is porphine, a rare chemical compound of exclusively theoretical interest. Substituted porphines are called porphyrins.[1] With a total of 26 π-electrons, of which 18 π-electrons form a planar, continuous cycle, the porphyrin ring structure is often described as aromatic.[2][3] One result of the large conjugated system is that porphyrins typically absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored. The name "porphyrin" derives from the Greek word πορφύρα (porphyra), meaning purple.[4]
Structure
Porphyrin complexes consist of a square planar MN4 core. The periphery of the porphyrins, consisting of sp2-hybridized carbons, generally display small deviations from planarity. "Ruffled" or saddle-shaped porphyrins is attributed to interactions of the system with its environment.[5] Additionally, the metal is often not centered in the N4 plane.[6] For free porphyrins, the two pyrrole protons are mutually trans and project out of the N4 plane.[7] These nonplanar distortions are associated with altered chemical and physical properties. Chlorophyll-rings are more distinctly nonplanar, but they are more saturated than porphyrins.[8]
Complexes of porphyrins
Concomitant with the displacement of two N-H protons, porphyrins bind metal ions in the N4 "pocket". The metal ion usually has a charge of 2+ or 3+. A schematic equation for these syntheses is shown:
- H2porphyrin + [MLn]2+ → M(porphyrinate)Ln−4 + 4 L + 2 H+, where M = metal ion and L = a ligand
- Representative porphyrins and derivatives
- Derivatives of protoporphyrin IX are common in nature, the precursor to hemes.
- Octaethylporphyrin (H2OEP) is a synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, OEP2− is highly symmetrical.
- Tetraphenylporphyrin (H2TPP)is another synthetic analogue of protoporphyrin IX. Unlike the natural porphyrin ligands, TPP2− is highly symmetrical. Another difference is that its methyne centers are occupied by phenyl groups.
- Simplified view of heme, a complex of a protoporphyrin IX.
- A macrocycle of 40 porphyrin molecules, model
- A macrocycle of 40 porphyrin molecules, STM image
Ancient porphyrins
A geoporphyrin, also known as a petroporphyrin, is a porphyrin of geologic origin.[9] They can occur in crude oil, oil shale, coal, or sedimentary rocks.[9][10] Abelsonite is possibly the only geoporphyrin mineral, as it is rare for porphyrins to occur in isolation and form crystals.[11]
The field of organic geochemistry had its origins in the isolation of porphyrins from petroleum.[citation needed] This finding helped establish the biological origins of petroleum. Petroleum is sometimes "fingerprinted" by analysis of trace amounts of nickel and vanadyl porphyrins.[citation needed]
Biosynthesis
In non-photosynthetic eukaryotes such as animals, insects, fungi, and protozoa, as well as the α-proteobacteria group of bacteria, the committed step for porphyrin biosynthesis is the formation of δ-aminolevulinic acid (δ-ALA, 5-ALA or dALA) by the reaction of the amino acid glycine with succinyl-CoA from the citric acid cycle. In plants, algae, bacteria (except for the α-proteobacteria group) and archaea, it is produced from glutamic acid via glutamyl-tRNA and glutamate-1-semialdehyde. The enzymes involved in this pathway are glutamyl-tRNA synthetase, glutamyl-tRNA reductase, and glutamate-1-semialdehyde 2,1-aminomutase. This pathway is known as the C5 or Beale pathway.
Two molecules of dALA are then combined by porphobilinogen synthase to give porphobilinogen (PBG), which contains a pyrrole ring. Four PBGs are then combined through deamination into hydroxymethyl bilane (HMB), which is hydrolysed to form the circular tetrapyrrole uroporphyrinogen III. This molecule undergoes a number of further modifications. Intermediates are used in different species to form particular substances, but, in humans, the main end-product protoporphyrin IX is combined with iron to form heme. Bile pigments are the breakdown products of heme.
The following scheme summarizes the biosynthesis of porphyrins, with references by EC number and the OMIM database. The porphyria associated with the deficiency of each enzyme is also shown:

Laboratory synthesis

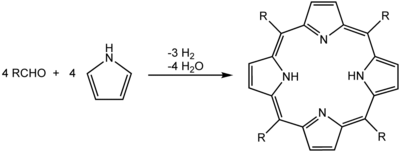
A common synthesis for porphyrins is the Rothemund reaction, first reported in 1936,[12][13] which is also the basis for more recent methods described by Adler and Longo.[14] The general scheme is a condensation and oxidation process starting with pyrrole and an aldehyde.
Potential applications
Photodynamic therapy
Porphyrins have been evaluated in the context of photodynamic therapy (PDT) since they strongly absorb light, which is then converted to heat in the illuminated areas.[15] This technique has been applied in macular degeneration using verteporfin.[16]
PDT is considered a noninvasive cancer treatment, involving the interaction between light of a determined frequency, a photo-sensitizer, and oxygen. This interaction produces the formation of a highly reactive oxygen species (ROS), usually singlet oxygen, as well as superoxide anion, free hydroxyl radical, or hydrogen peroxide.[17] These high reactive oxygen species react with susceptible cellular organic biomolecules such as; lipids, aromatic amino acids, and nucleic acid heterocyclic bases, to produce oxidative radicals that damage the cell, possibly inducing apoptosis or even necrosis.[18]
Molecular electronics and sensors
Porphyrin-based compounds are of interest as possible components of molecular electronics and photonics.[19] Synthetic porphyrin dyes have been incorporated in prototype dye-sensitized solar cells.[20][21]
Biological applications
Porphyrins have been investigated as possible anti-inflammatory agents[22] and evaluated on their anti-cancer and anti-oxidant activity.[23] Several porphyrin-peptide conjugates were found to have antiviral activity against HIV in vitro.[24]
Toxicology
Heme biosynthesis is used as biomarker in environmental toxicology studies. While excess production of porphyrins indicate organochlorine exposure, lead inhibits ALA dehydratase enzyme.[25]
Gallery
- Lewis structure for meso-tetraphenylporphyrin
- UV–vis readout for meso-tetraphenylporphyrin
- Light-activated porphyrin. Monatomic oxygen. Cellular aging
Related species
In nature
Several heterocycles related to porphyrins are found in nature, almost always bound to metal ions. These include
| N4-macrocycle | Cofactor name | metal | comment |
|---|---|---|---|
| chlorin | chlorophyll | magnesium | several versions of chlorophyll exist (sidechain; exception being chlorophyll c) |
| bacteriochlorin | bacteriochlorophyll (in part) | magnesium | several versions of bacteriochlorophyll exist (sidechain; some use a usual chlorin ring) |
| sirohydrochlorin (an isobacteriochlorin) | siroheme | iron | Important cofactor in sulfur assimilation |
| biosynthetic intermediate en route to cofactor F430 and B12 | |||
| corrin | vitamin B12 | cobalt | several variants of B12 exist (sidechain) |
| corphin | Cofactor F430 | nickel | highly reduced macrocycle |
Synthetic
A benzoporphyrin is a porphyrin with a benzene ring fused to one of the pyrrole units. e.g. verteporfin is a benzoporphyrin derivative.[26]
Non-natural porphyrin isomers

The first synthetic porphyrin isomer was reported by Emanual Vogel and coworkers in 1986.[27] This isomer [18]porphyrin-(2.0.2.0) is named as porphycene, and the central N4 Cavity forms a rectangle shape as shown in figure.[28] Porphycenes showed interesting photophysical behavior and found versatile compound towards the photodynamic therapy.[29] This inspired Vogel and Sessler to took up the challenge of preparing [18]porphyrin-(2.1.0.1) and named it as corrphycene or porphycerin.[30] The third porphyrin that is [18]porphyrin-(2.1.1.0), was reported by Callot and Vogel-Sessler. Vogel and coworkers reported successful isolation of [18]porphyrin-(3.0.1.0) or isoporphycene.[31] The Japanese scientist Furuta[32] and Polish scientist Latos-Grażyński[33] almost simultaneously reported the N-confused porphyrins. The inversion of one of the pyrrolic subunits in the macrocyclic ring resulted in one of the nitrogen atoms facing outwards from the core of the macrocycle.

See also
- A porphyrin-related disease: porphyria
- Porphyrin coordinated to iron: heme
- A heme-containing group of enzymes: Cytochrome P450
- Porphyrin coordinated to magnesium: chlorophyll
- The one-carbon-shorter analogues: corroles, including vitamin B12, which is coordinated to a cobalt
- Corphins, the highly reduced porphyrin coordinated to nickel that binds the Cofactor F430 active site in methyl coenzyme M reductase (MCR)
- Nitrogen-substituted porphyrins: phthalocyanine
References
External links