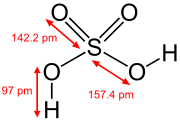
Sulphuric acid
Sulphuric acid (alternative spellin sulfuric acid) is a heichly corrosive strang mineral acid wi the molecular formula H2SO4.
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name Sulfuric acid | |||
| Ither names Ile o vitriol | |||
| Identifiers | |||
CAS Nummer | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Nummer | 231-639-5 | ||
| KEGG | |||
| RTECS nummer | WS5600000 | ||
| UNII | |||
| UN nummer | 1830 | ||
| |||
SMILES
| |||
| Properties | |||
| H 2SO 4 | |||
| Molar mass | 98.079 g/mol | ||
| Appearance | Clear, colourless, odorless liquid | ||
| Density | 1.84 g/cm3, liquid | ||
| Meltin pynt | 10 °C (50 °F; 283 K) | ||
| Bylin pynt | 337 °C (639 °F; 610 K) | ||
Solubility in watter | miscible | ||
| Acidity (pKa) | −3, 1.99 | ||
| Viscosity | 26.7 cP (20 °C) | ||
| Thermochemistry | |||
| Staundart molar entropy S | 157 J·mol−1·K−1[1] | ||
| Std enthalpy o formation ΔfH | −814 kJ·mol−1[1] | ||
| Hazards | |||
| GHS pictograms |  | ||
| GHS signal wird | Danger | ||
| GHS hazard statements | H314 | ||
| GHS precautionary statements | P260, P264, P280, P301+330+331, P303+361+353, P363, P304+340, P305+351+338, P310, P321, P310, P405, P501 | ||
| NFPA 704 |  0 3 2 | ||
| Flash pynt | Non-flammable | ||
| Threshold Leemit Value | 15 mg/m3 (IDLH), 1 mg/m3 (TWA), 2 mg/m3 (STEL) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (Median dose) | 2140 mg/kg (oral, rat), LC50 = 25 mg/m3 (inhalation, rat) | ||
| Relatit compoonds | |||
Except whaur itherwise notit, data are gien for materials in thair staundart state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
References
🔥 Top keywords: Main PageAshley StorrieCarles PuigdemontMarie AntoinetteSpecial:RecentChangesSpecial:SearchWikipedia:Commonty YettWikipedia:Contact usJaney GodleyScots leidFile:Nvidia logo.svgFile:KFC logo.svgWarld War IISuzuki EsteemWikipedia:AbootThe Lion KingFile:FC Barcelona (crest).svgHelp:ContentsFacebookKFCFile:Starbucks Corporation Logo 2011.svgFile:Manchester City FC badge.svgConstellationWikipedia:General disclamationPortal:Current eventsWikipaediaToyota AgyaSpecial:MyTalkWikipedia:Mercat CrossContact lensWikipedia:Apryle 2024 Writin DriveFile:Real Madrid CF.svgThe Selkirk GraceChief executive officerUiser collogue:AmaryllisGardenerGujaratWikipedia:Walcome, ane an awScotlandJordan Frieda

