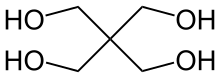
Pentaerythritol is an organic compound with the formula C(CH2OH)4. Classified as a polyol, it is a white solid. Pentaerythritol is a building block for the synthesis and production of explosives, plastics, paints, appliances, cosmetics, and many other commercial products.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 2,2-Bis(hydroxymethyl)propane-1,3-diol[1] | |
| Other names 2,2-Bis(hydroxymethyl)1,3-propanediol Pentaerythritol[1] Hercules P 6 Monopentaerythritol Tetramethylolmethane THME PETP Pentaerythrite Pentek Hercules Aqualon improved technical PE-200 | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.732 |
| EC Number |
|
| KEGG | |
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C5H12O4 | |
| Molar mass | 136.15 g/mol |
| Appearance | white solid |
| Density | 1.396 g/cm3 |
| Melting point | 260.5 °C (500.9 °F; 533.6 K) |
| Boiling point | 276 °C (529 °F; 549 K) at 30 mmHg |
| |
| Solubility | Slightly soluble in:methanol, ethanol, glycerol, ethylene glycol, formamide; insoluble in: acetone, toluene, heptane, diethyl ether, dichloromethane |
| Vapor pressure | 0.00000008 mmHg (20°C)[4] |
| Hazards | |
| Flash point | 200.1 °C (392.2 °F; 473.2 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) | TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[4] |
REL (Recommended) | TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[4] |
IDLH (Immediate danger) | N.D.[4] |
| Related compounds | |
Related compounds | Neopentane, Neopentyl alcohol, Neopentyl glycol, Trimethylolethane, Orthocarbonic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
The word pentaerythritol is a blend of penta- in reference to its 5 carbon atoms and erythritol, which also possesses 4 alcohol groups.
Synthesis
Pentaerythritol was first reported in 1891 by German chemist Bernhard Tollens and his student P. Wigand.[5] It may be prepared via a base-catalyzed multiple-addition reaction between acetaldehyde and 3 equivalents of formaldehyde to give pentaerythrose (CAS: 3818-32-4), followed by a Cannizzaro reaction with a fourth equivalent of formaldehyde to give the final product plus formate ion.[6]
Uses
Pentaerythritol is a versatile building block for the preparation of many compounds,[7] particularly polyfunctionalized derivatives. applications include alkyd resins, varnishes, polyvinyl chloride stabilizers, tall oil esters, antioxidants (e.g. Anox 20). Such derivatives are found in plastics, paints, cosmetics, and many other products.[8]
Esters of pentaerythitol are biodegradable,[9][10] and they are used as transformer oils.[11] Due to a very high flash point they also find some use in lubricating gas turbines.[12]
Polyester derivatives
Pentaerythritol is a precursor to esters of the type C(CH2OX)4. Such derivatives are pentaerythritol tetranitrate (PETN), a vasodilator and explosive, the trinitrate derivative pentrinitrol (Petrin), the tetraacetate normosterol (PAG), and the polymer cross-linking agents pentaerythritol tetraacrylate and pentaerythritol tetrakis(3-mercaptopropionate).[13][14]
Fire retardants
Pentaerythritol is used as a fire retardant, such as in plastics and intumescent paints and coatings. It releases water upon heating and leaves a deposit of thermally insulating char.[15]
