International System of Units
The International System of Units is the standard modern form of the metric system. The name of this system can be shortened or abbreviated to SI, from the French name Système International d'unités.
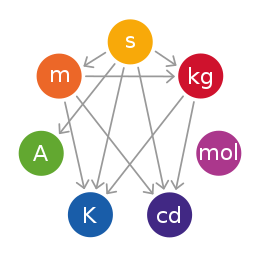
The International System of Units is a system of measurement based on 7 base units: the metre (length), kilogram (mass), second (time), ampere (electric current), kelvin (temperature), mole (quantity), and candela (brightness). These base units can be used in combination with each other. This creates SI derived units, which can be used to describe other quantities, such as volume, energy, pressure, and velocity.
The system is used almost globally. Only Myanmar, Liberia, and the United States do not use SI as their official system of measurement.[1] In these countries, though, SI is commonly used in science and medicine.
History and use
The metric system was created in France after the French Revolution in 1789. The original system only had two standard units, the kilogram and the metre. The metric system became popular amongst scientists.
In the 1860s, James Clerk Maxwell and William Thomson (later known as Lord Kelvin) suggested a system with three base units – length, mass, and time. Other units would be derived from those three base units. Later, this suggestion would be used to create the centimetre-gram-second system of units (CGS), which used the centimetre as the base unit for length, the gram as the base unit for mass, and the second as the base unit for time. It also added the dyne as the base unit for force and the erg as the base unit for energy.
As scientists studied electricity and magnetism, they realized other base units were needed to describe these subjects. By the middle of the 20th century, many different versions of the metric system were being used. This was very confusing.
In 1954, the 9th General Conference on Weights and Measures (CGPM) created the first version of the International System of Units. The six base units that they used were the metre, kilogram, second, ampere, Kelvin, and candela.[2] The seventh base unit, the mole, was added in 1971.[3]
SI is now used almost everywhere in the world, except in the United States, Liberia and Myanmar, where the older imperial units are still widely used. Other countries, most of them historically related to the British Empire, are slowly replacing the old imperial system with the metric system or using both systems at the same time.
Units of measurement
Base units
The SI base units are measurements used by scientists and other people around the world. All the other units can be written by combining these seven base units in different ways. These other units are called "derived units".
SI base units[4]: 23 [5][6] Unit
nameUnit
symbolDimension
symbolQuantity
nameDefinition[n 1] second s T time - Prior:
of a day of 24 hours of 60 minutes of 60 seconds
- Interim (1956):
of the tropical year for 1900 January 0 at 12 hours ephemeris time.
- Current (1967): The duration of 9192631770 periods of the radiation corresponding to the transition between the two hyperfine levels of the ground state of the caesium-133 atom.
metre m L length - Prior (1793):
of the meridian through Paris between the North Pole and the Equator.FG
- Interim (1960): 1650763.73 wavelengths in a vacuum of the radiation corresponding to the transition between the 2p10 and 5d5 quantum levels of the krypton-86 atom.
- Current (1983): The distance travelled by light in vacuum in
second.
kilogram
[n 2]kg M mass - Prior (1793): The grave was defined as being the mass (then called weight) of one litre of pure water at its freezing point.FG
- Interim (1889): The mass of a small squat cylinder of ~47 cubic centimetres of platinum-iridium alloy kept in the International Bureau of Weights and Measures, Paris, France.[7]
- Current (2019): The kilogram is defined by setting the Planck constant h exactly to 6.62607015×10−34 J⋅s (J = kg⋅m2⋅s−2), given the definitions of the metre and the second.[8] Then the formula would be 1 kg = h⁄6.62607015 × 10-34⋅m2⋅s−1
ampere A I electric current - Prior (1881): A tenth of the electromagnetic CGS unit of current. The [CGS] electromagnetic unit of current is that current, flowing in an arc 1 cm long of a circle 1 cm in radius, that creates a field of one oersted at the centre.[9] IEC
- Interim (1946): The constant current which, if maintained in two straight parallel conductors of infinite length, of negligible circular cross-section, and placed 1 m apart in vacuum, would produce between these conductors a force equal to 2×10−7 newtons per metre of length.
- Current (2019): The flow of 1⁄1.602176634×10−19 times the elementary charge e per second.
kelvin K Θ thermodynamic
temperature- Prior (1743): The centigrade scale is obtained by assigning 0 °C to the freezing point of water and 100 °C to the boiling point of water.
- Interim (1954): The triple point of water (0.01 °C) defined to be exactly 273.16 K.[n 3]
- Previous (1967):
of the thermodynamic temperature of the triple point of water.
- Current (2019): The kelvin is defined by setting the fixed numerical value of the Boltzmann constant k to 1.380649×10−23 J⋅K−1, (J = kg⋅m2⋅s−2), given the definition of the kilogram, the metre and the second.
mole mol N amount of
substance- Prior (1900): A stoichiometric quantity which is the equivalent mass in grams of Avogadro's number of molecules of a substance.ICAW
- Interim (1967): The amount of substance of a system which contains as many elementary entities[n 4] as there are atoms in 0.012 kilogram of carbon-12.
- Current (2019): The amount of substance of exactly 6.02214076×1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol−1 and is called the Avogadro number.
candela cd J luminous
intensity- Prior (1946): The value of the new candle (early name for the candela) is such that the brightness of the full radiator at the temperature of solidification of platinum is 60 new candles per square centimetre.
- Current (1979): The luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency 5.4×1014 hertz and that has a radiant intensity in that direction of
watt per steradian.
- Note: both old and new definitions are approximately the luminous intensity of a whale blubber candle burning modestly bright, in the late 19th century called a "candlepower" or a "candle".
- Notes
The Prior definitions of the various base units in the above table were made by the following authorities:
- FG = French Government
- IEC = International Electrotechnical Commission
- ICAW = International Committee on Atomic Weights
All other definitions result from resolutions by either CGPM or the CIPM and are catalogued in the SI Brochure.
- Prior:
Derived units
Derived units are created by combining the base units. The base units can be divided, multiplied, or raised to powers. Some derived units have special names. Usually these were created to make calculations simpler.
| Name | Symbol | Quantity | Definition other units | Definition SI base units |
|---|---|---|---|---|
| radian | rad | plane angle | − | |
| steradian | sr | solid angle | − | |
| hertz | Hz | frequency | s−1 | |
| newton | N | force, weight | m⋅kg⋅s−2 | |
| pascal | Pa | pressure, stress | N/m2 | m−1⋅kg⋅s−2 |
| joule | J | energy, work, heat | N⋅m | m2⋅kg⋅s−2 |
| watt | W | power, radiant flux | J/s | m2⋅kg⋅s−3 |
| coulomb | C | electric charge | s⋅A | |
| volt | V | voltage, electrical potential difference, electromotive force | W/A J/C | m2⋅kg⋅s−3⋅A−1 |
| farad | F | electrical capacitance | C/V | m−2⋅kg−1⋅s4⋅A2 |
| ohm | Ω | electrical resistance, impedance, reactance | V/A | m2⋅kg⋅s−3⋅A−2 |
| siemens | S | electrical conductance | 1/Ω | m−2⋅kg−1⋅s3⋅A2 |
| weber | Wb | magnetic flux | J/A | m2⋅kg⋅s−2⋅A−1 |
| tesla | T | magnetic field strength | Wb/m2 V⋅s/m2 N/(A⋅m) | kg⋅s−2⋅A−1 |
| henry | H | inductance | Wb/A V⋅s/A | m2⋅kg⋅s−2⋅A−2 |
| degree Celsius | °C | temperature relative to 273.15 K | TK − 273.15 | K |
| lumen | lm | luminous flux | cd⋅sr | cd |
| lux | lx | illuminance | lm/m2 | m−2⋅cd |
| becquerel | Bq | radioactivity (decays per unit time) | s−1 | |
| gray | Gy | absorbed dose (of ionizing radiation) | J/kg | m2⋅s−2 |
| sievert | Sv | equivalent dose (of ionizing radiation) | J/kg | m2⋅s−2 |
| katal | kat | catalytic activity | s−1⋅mol |
Prefixes
Very large or very small measurements can be written using prefixes. Prefixes are added to the beginning of the unit to make a new unit. For example, the prefix kilo- means "1000" times the original unit and the prefix milli- means "0.001" times the original unit. So one kilometre is 1000 metres and one milligram is a 1000th of a gram.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||